Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial
- PMID: 15753541
- PMCID: PMC1774430
- DOI: 10.1136/gut.2004.047563
Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial
Abstract
Background: Proinflammatory cytokines, especially tumour necrosis factor alpha (TNF-alpha), play a prominent role in the pathogenesis of cancer cachexia. Thalidomide, which is an inhibitor of TNF-alpha synthesis, may represent a novel and rational approach to the treatment of cancer cachexia.
Aims: To assess the safety and efficacy of thalidomide in attenuating weight loss in patients with cachexia secondary to advanced pancreatic cancer.
Methods: Fifty patients with advanced pancreatic cancer who had lost at least 10% of their body weight were randomised to receive thalidomide 200 mg daily or placebo for 24 weeks in a single centre, double blind, randomised controlled trial. The primary outcome was change in weight and nutritional status.
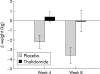
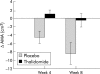
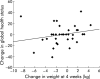
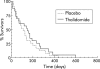
Results: Thirty three patients (16 control, 17 thalidomide) were evaluated at four weeks, and 20 patients (eight control, 12 thalidomide) at eight weeks. At four weeks, patients who received thalidomide had gained on average 0.37 kg in weight and 1.0 cm(3) in arm muscle mass (AMA) compared with a loss of 2.21 kg (absolute difference -2.59 kg (95% confidence interval (CI) -4.3 to -0.8); p = 0.005) and 4.46 cm(3) (absolute difference -5.6 cm(3) (95% CI -8.9 to -2.2); p = 0.002) in the placebo group. At eight weeks, patients in the thalidomide group had lost 0.06 kg in weight and 0.5 cm(3) in AMA compared with a loss of 3.62 kg (absolute difference -3.57 kg (95% CI -6.8 to -0.3); p = 0.034) and 8.4 cm(3) (absolute difference -7.9 cm(3) (95% CI -14.0 to -1.8); p = 0.014) in the placebo group. Improvement in physical functioning correlated positively with weight gain (r = 0.56, p = 0.001).
Conclusion: Thalidomide was well tolerated and effective at attenuating loss of weight and lean body mass in patients with cachexia due to advanced pancreatic cancer.
Figures

Comment in
-
Thalidomide and cancer cachexia: old problem, new hope?Gut. 2005 Apr;54(4):447-8. doi: 10.1136/gut.2004.053330. Gut. 2005. PMID: 15753523 Free PMC article. Review. No abstract available.
Similar articles
-
Poor tolerability of thalidomide in end-stage oesophageal cancer.Eur J Cancer Care (Engl). 2011 Sep;20(5):593-600. doi: 10.1111/j.1365-2354.2011.01255.x. Epub 2011 Apr 27. Eur J Cancer Care (Engl). 2011. PMID: 21521389 Clinical Trial.
-
The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study.J Palliat Med. 2012 Oct;15(10):1059-64. doi: 10.1089/jpm.2012.0146. Epub 2012 Aug 10. J Palliat Med. 2012. PMID: 22880820 Free PMC article. Clinical Trial.
-
Oesophageal cancer and cachexia: the effect of short-term treatment with thalidomide on weight loss and lean body mass.Aliment Pharmacol Ther. 2003 Mar 1;17(5):677-82. doi: 10.1046/j.1365-2036.2003.01457.x. Aliment Pharmacol Ther. 2003. PMID: 12641516 Clinical Trial.
-
Thalidomide and cancer cachexia: old problem, new hope?Gut. 2005 Apr;54(4):447-8. doi: 10.1136/gut.2004.053330. Gut. 2005. PMID: 15753523 Free PMC article. Review. No abstract available.
-
Exercise for cancer cachexia in adults.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD010804. doi: 10.1002/14651858.CD010804.pub3. Cochrane Database Syst Rev. 2021. PMID: 33735441 Free PMC article.
Cited by
-
Understanding the mechanisms and treatment options in cancer cachexia.Nat Rev Clin Oncol. 2013 Feb;10(2):90-9. doi: 10.1038/nrclinonc.2012.209. Epub 2012 Dec 4. Nat Rev Clin Oncol. 2013. PMID: 23207794 Review.
-
Disrupting cytokine signaling in pancreatic cancer: a phase I/II study of etanercept in combination with gemcitabine in patients with advanced disease.Pancreas. 2013 Jul;42(5):813-8. doi: 10.1097/MPA.0b013e318279b87f. Pancreas. 2013. PMID: 23429495 Free PMC article. Clinical Trial.
-
Nutritional status and nutritional support before and after pancreatectomy for pancreatic cancer and chronic pancreatitis.Indian J Surg Oncol. 2012 Dec;3(4):348-59. doi: 10.1007/s13193-012-0189-4. Epub 2012 Oct 30. Indian J Surg Oncol. 2012. PMID: 24293974 Free PMC article. Review.
-
Cancer cachexia prevention via physical exercise: molecular mechanisms.J Cachexia Sarcopenia Muscle. 2013 Jun;4(2):111-24. doi: 10.1007/s13539-012-0096-0. Epub 2012 Dec 13. J Cachexia Sarcopenia Muscle. 2013. PMID: 23239116 Free PMC article.
-
Thalidomide in dermatology: revisited.Indian J Dermatol. 2015 Mar-Apr;60(2):213. doi: 10.4103/0019-5154.152580. Indian J Dermatol. 2015. PMID: 25814738 Free PMC article.
References
-
- Dunlop R. Clinical epidemiology of cancer cachexia. In: Bruera E, Higginson I, eds. Cachexia-anorexia in cancer patients, vol 5. Oxford: Oxford University Press, 1996:76–82.
-
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–71. - PubMed
-
- Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol 1999;8:133–41. - PubMed
-
- Oliff A, Defeo-Jones D, Boyer M, et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 1987;50:555–63. - PubMed
-
- Langstein HN, Doherty GM, Fraker DL, et al. The roles of gamma-interferon and tumor necrosis factor alpha in an experimental rat model of cancer cachexia. Cancer Res 1991;51:2302–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
