Curcumin modulation of Na,K-ATPase: phosphoenzyme accumulation, decreased K+ occlusion, and inhibition of hydrolytic activity
- PMID: 15753945
- PMCID: PMC1576134
- DOI: 10.1038/sj.bjp.0706185
Curcumin modulation of Na,K-ATPase: phosphoenzyme accumulation, decreased K+ occlusion, and inhibition of hydrolytic activity
Abstract
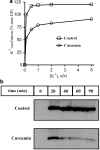
1 Curcumin, the major constitute of tumeric, is an important nutraceutical that has been shown to be useful in the treatment of many diseases. As an inhibitor of the sarcoplasmic reticulum Ca(2+)-ATPase, curcumin was shown to correct cystic fibrosis (CF) defects in some model systems, whereas others have reported no or little effects on CF after curcumin treatment, suggesting that curcumin effect is not due to simple inhibition of the Ca(2+)-ATPase. 2 We tested the hypothesis that curcumin may modulate other members of the P(2)-type ATPase superfamily by studying the effects of curcumin on the activity and kinetic properties of the Na,K-ATPase. 3 Curcumin treatment inhibited Na,K-ATPase activity in a dose-dependent manner (K(0.5) approximately 14.6 microM). Curcumin decreased the apparent affinity of Na,K-ATPase for K(+) and increased it for Na(+) and ATP. Kinetic analyses indicated that curcumin induces a three-fold reduction in the rate of E1P --> E2P transition, thereby increasing the steady-state phosphoenzyme level. Curcumin treatment significantly abrogated K(+) occlusion to the enzyme as evidenced from kinetic and proteolytic cleavage experiments. Curcumin also significantly decreased the vanadate sensitivity of the enzyme. 4 Thus, curcumin partially blocks the K(+) occlusion site, and induces a constitutive shift in the conformational equilibrium of the enzyme, towards the E1 conformation. 5 The physiological consequences of curcumin treatment previously reported in different epithelial model systems may, at least in part, be related to the direct effects of curcumin on Na,K-ATPase activity.
Figures






Similar articles
-
Leucine 332 at the boundary between the fourth transmembrane segment and the cytoplasmic domain of Na+,K+-ATPase plays a pivotal role in the ion translocating conformational changes.Biochemistry. 1997 Oct 28;36(43):13312-24. doi: 10.1021/bi971030q. Biochemistry. 1997. PMID: 9341223
-
Functional significance of the shark Na,K-ATPase N-terminal domain. Is the structurally variable N-Terminus involved in tissue-specific regulation by FXYD proteins?Biochemistry. 2005 Oct 4;44(39):13051-62. doi: 10.1021/bi0504456. Biochemistry. 2005. PMID: 16185073
-
Interaction of ATP with the phosphoenzyme of the Na+,K+-ATPase.Biochemistry. 2010 Feb 16;49(6):1248-58. doi: 10.1021/bi9019548. Biochemistry. 2010. PMID: 20063899
-
Mutant Phe788 --> Leu of the Na+,K+-ATPase is inhibited by micromolar concentrations of potassium and exhibits high Na+-ATPase activity at low sodium concentrations.Biochemistry. 1999 Aug 31;38(35):11389-400. doi: 10.1021/bi990951t. Biochemistry. 1999. PMID: 10471289
-
Role of protein conformation changes and transphosphorylations in the function of Na+/K(+)-transporting adenosine triphosphatase: an attempt at an integration into the Na+/K+ pump mechanism.Biol Rev Camb Philos Soc. 1992 Feb;67(1):31-78. doi: 10.1111/j.1469-185x.1992.tb01658.x. Biol Rev Camb Philos Soc. 1992. PMID: 1318758 Review.
Cited by
-
Modulation of protein kinase C by curcumin; inhibition and activation switched by calcium ions.Br J Pharmacol. 2007 Jan;150(2):200-8. doi: 10.1038/sj.bjp.0706970. Epub 2006 Dec 11. Br J Pharmacol. 2007. PMID: 17160011 Free PMC article.
-
Capsazepine, a synthetic vanilloid that converts the Na,K-ATPase to Na-ATPase.Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1757-61. doi: 10.1073/pnas.0711838105. Epub 2008 Jan 29. Proc Natl Acad Sci U S A. 2008. PMID: 18230728 Free PMC article.
-
Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats.Free Radic Res. 2015 Mar;49(3):279-89. doi: 10.3109/10715762.2014.999674. Epub 2015 Jan 28. Free Radic Res. 2015. PMID: 25536420 Free PMC article.
-
Circular RNA and intervertebral disc degeneration: unravelling mechanisms and implications.Front Mol Biosci. 2023 Dec 19;10:1302017. doi: 10.3389/fmolb.2023.1302017. eCollection 2023. Front Mol Biosci. 2023. PMID: 38192334 Free PMC article. Review.
-
The modulation of erythrocyte Na(+)/K(+)-ATPase activity by curcumin.J Adv Res. 2015 Nov;6(6):1023-30. doi: 10.1016/j.jare.2014.12.007. Epub 2015 Jan 22. J Adv Res. 2015. PMID: 26644941 Free PMC article.
References
-
- ANDERSEN J.P., LASSEN K., MØLLER J.V. Changes in Ca2+ affinity related to conformational transitions in the phosphorylated state of soluble monomeric Ca2+-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 1985;260:371–380. - PubMed
-
- ARATO-OSHIMA T., MATSUI H., WAKIZAKA A., HOMAREDA H. Mechanism responsible for oligomycin-induced occlusion of Na+ within Na/K-ATPase. J. Biol. Chem. 1996;271:25604–25610. - PubMed
-
- BILMEN J.G., KHAN S.Z., JAVED M., MICHELANGELI F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur. J. Biochem. 2001;268:6318–6327. - PubMed
-
- CHENG A.L., HSU C.H., LIN J.K., HSU M.M., HO Y.F., SHEN T.S., KO J.Y., LIN J.T., LIN B.R., MING-SHIANG W., YU H.S., JEE S.H., CHEN G.S., CHEN T.M., CHEN C.A., LAI M.K., PU Y.S., PAN M.H., WANG Y.J., TSAI C.C., HSIEH C.Y. Phase 1 clinical trial of curcumin, a chemopreventive agent, in patients with high-risk of pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

