Enhanced fluorescence cyanide detection at physiologically lethal levels: reduced ICT-based signal transduction
- PMID: 15755185
- PMCID: PMC6844257
- DOI: 10.1021/ja044421i
Enhanced fluorescence cyanide detection at physiologically lethal levels: reduced ICT-based signal transduction
Abstract
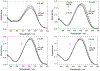
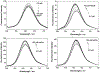
Three water-soluble fluorescent probes have been specifically designed to determine free cyanide concentrations up to physiologically lethal levels, >20 microM. The probes have been designed in such a way as to afford many notable sensing features, which render them unique with regard to signal transduction, photophysical characteristics, and their application to physiological cyanide determination and safeguard. The probes are readily able to reversibly bind free aqueous cyanide with dissociation constants around 4 microM3. Subsequent cyanide binding modulates the intramolecular charge transfer within the probes, a change in the electronic properties within the probes, resulting in enhanced fluorescence optical signals as a function of increased solution cyanide concentration. The ground-state chelation with cyanide produces wavelength shifts, which also enable the probes to sense cyanide in both an excitation and emission ratiometric manner, in addition to enhanced fluorescence signaling. This has enabled a generic cyanide sensing platform to be realized that is not dependent on fluorescent probe concentration, probe photodegradation, or fluctuations in the intensity of any employed excitation sources, ideal for remote cyanide sensing applications. Further, the >600 nm fluorescence emission of the probes potentially allows for enhanced fluorescence ratiometric cyanide sensing in the optical window of tissues and blood, facilitating their use for the transdermal monitoring of cyanide for mammalian safeguard or postmortem in fire victims, both areas of active research.
Figures



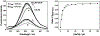


References
-
- Baskin SI; Brewer TG Medical Aspects of Chemical and Biological Warfare; Sidell F, Takafuji ET, Franz DR, Eds.; TMM Publications: Washington, DC, 1997; Chapter 10, pp 271–286 and references therein.
-
- Warburg O Hoppe-Seyler’s Z. Physiol. Chem 1911, 76, 331–346.
-
- Kellin D Proc. R. Soc. London, Ser. B: Biol. Sci 1929, 104, 206–251.
-
- Ishii A; Seno H; Watanabe-Suzuki K; Suzuki O; Kumazawa T Anal. Chem 1998, 70 (22), 4873–4876. - PubMed
-
- Moriva F; Hashimoto YJ For. Sci 2001, 46 (6), 1421–1425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

