Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo
- PMID: 15755727
- PMCID: PMC1283069
- DOI: 10.1074/jbc.M501757200
Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo
Abstract
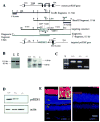
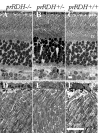
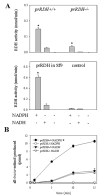
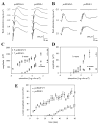
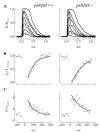
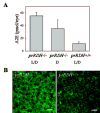
The retinoid cycle is a recycling system that replenishes the 11-cis-retinal chromophore of rhodopsin and cone pigments. Photoreceptor-specific retinol dehydrogenase (prRDH) catalyzes reduction of all-trans-retinal to all-trans-retinol and is thought to be a key enzyme in the retinoid cycle. We disrupted mouse prRDH (human gene symbol RDH8) gene expression by targeted recombination and generated a homozygous prRDH knock-out (prRDH-/-) mouse. Histological analysis and electron microscopy of retinas from 6- to 8-week-old prRDH-/- mice revealed no structural differences of the photoreceptors or inner retina. For brief light exposure, absence of prRDH did not affect the rate of 11-cis-retinal regeneration or the decay of Meta II, the activated form of rhodopsin. Absence of prRDH, however, caused significant accumulation of all-trans-retinal following exposure to bright lights and delayed recovery of rod function as measured by electroretinograms and single cell recordings. Retention of all-trans-retinal resulted in slight overproduction of A2E, a condensation product of all-trans-retinal and phosphatidylethanolamine. We conclude that prRDH is an enzyme that catalyzes reduction of all-trans-retinal in the rod outer segment, most noticeably at higher light intensities and prolonged illumination, but is not an essential enzyme of the retinoid cycle.
Figures



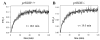

References
-
- Kuksa V, Imanishi Y, Batten M, Palczewski K, Moise AR. Vision Res. 2003;43:2959–2981. - PubMed
-
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Prog Retin Eye Res. 2001;20:469–529. - PubMed
-
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. Nat Genet. 1997;15:236–246. - PubMed
-
- Sun H, Molday RS, Nathans J. J Biol Chem. 1999;274:8269–8281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

