Role of Toll-like receptors 2 and 4 in the induction of cyclooxygenase-2 in vascular smooth muscle
- PMID: 15755814
- PMCID: PMC555494
- DOI: 10.1073/pnas.0407655101
Role of Toll-like receptors 2 and 4 in the induction of cyclooxygenase-2 in vascular smooth muscle
Abstract
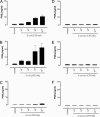
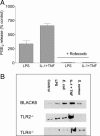
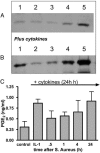
Bacteria stimulate macrophages as part of normal host defense. However, when this response is not limited, vascular smooth muscle may also be activated to express "vasoactive" genes (e.g., cyclooxygenase), leading to vascular collapse and septic shock. In macrophages, Toll-like receptors (TLRs) 4 and 2 transduce responses to Gram-negative and Gram-positive bacteria, respectively. However, the role of these TLRs in sensing bacteria in vascular smooth muscle is unclear. To address this question, we have cultured vascular smooth muscle cells from mice deficient in TLR4 (TLR4(-/-) mice), mice deficient in TLR2 (TLR2(-/-) mice), or control mice. Cells cultured from control or TLR2(-/-) mice, but not from TLR4(-/-) mice, expressed cyclooxygenase-2 and released increasing levels of prostaglandin E(2) after stimulation with whole Escherichia coli bacteria; the combination of IL-1beta plus TNF-alpha induced cyclooxygenase-2 in cells cultured from all three groups of animals. By contrast, Staphylococcus aureus affected cyclooxygenase-2 expression in two distinct ways. First, S. aureus induced a transient inhibition of cyclooxygenase-2 expression, which was overcome with time, and increased protein expression was noted. The effects of S. aureus on cyclooxygenase-2 expression were TLR2- and not TLR4-dependent. Thus, we show that Gram-positive and Gram-negative bacteria induce cyclooxygenase-2 in vascular smooth muscle with differing temporal profiles but with appropriate TLR2-versus-TLR4 signaling. These data have important implications for our understanding of the innate immune response in vascular cells and how it may impact vascular disease.
Figures


References
-
- Jourdan, K. B., Mitchell, J. A. & Evans, T. W. (1997) Biochem. Biophys. Res. Commun. 233, 668–672. - PubMed
-
- Bishop-Bailey, D., Pepper, J. R., Larkin, S. W. & Mitchell, J. A. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1655–1661. - PubMed
-
- Busse, R. & Mulsch A. (1990) FEBS Lett. 275, 87–90. - PubMed
-
- Woods, M., Bishop-Bailey, D., Pepper, J. R., Evans, T. W., Mitchell, J. A. & Warner, T. D. (1998) J. Cardiovasc. Pharmacol. 31, S348–S350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

