Saccharomyces cerevisiae Sps1p regulates trafficking of enzymes required for spore wall synthesis
- PMID: 15755916
- PMCID: PMC1087804
- DOI: 10.1128/EC.4.3.536-544.2005
Saccharomyces cerevisiae Sps1p regulates trafficking of enzymes required for spore wall synthesis
Abstract
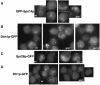
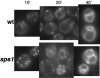
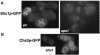
SPS1 encodes a sporulation-specific protein with homology to the Ste20/p21-activated kinase family. Deletion of SPS1 impinges on the formation of the spore wall, which surrounds each of the haploid nuclei generated by the meiotic divisions. Here, we demonstrate that the new internal membranes that surround the meiotic nuclei appear normal in the absence of Sps1p. Analyses of spore wall layers by immunohistochemistry suggest that the inner layers are not efficiently deposited. The defect in spore wall morphogenesis is most likely a consequence of mislocalization of enzymes required for the synthesis of the spore wall layers as both Chs3p, the major chitin synthase in yeast, and Gsc2/Fks2p, a glucan synthase transcriptionally upregulated during sporulation, fail to reach the prospore membrane in the sps1 mutant. Furthermore, localization of Chs3p to the prospore membrane is not dependent on Shc1p, a sporulation-specific homolog of Chs4p, which is required for recruitment of Chs3p to the bud neck in vegetative cells. Sps1p colocalized with Chs3p to peripheral and internal punctate structures and prospore membranes. We propose that Sps1p promotes sporulation, in part, by regulating the intracellular movement of proteins required for spore wall formation.
Figures




References
-
- Christodoulidou, A., V. Bouriotis, and G. Thireos. 1996. Two sporulation-specific chitin deacetylase-encoding genes are required for the ascospore wall rigidity of Saccharomyces cerevisiae. J. Biol. Chem. 271:31420-31425. - PubMed
-
- Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

