Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth
- PMID: 15755921
- PMCID: PMC1087799
- DOI: 10.1128/EC.4.3.588-603.2005
Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth
Abstract
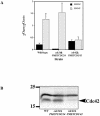
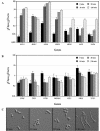
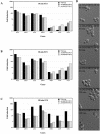
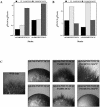
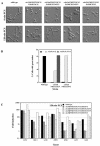
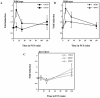
The Rho G protein Cdc42 and its exchange factor Cdc24 are required for hyphal growth of the human fungal pathogen Candida albicans. Previously, we reported that strains ectopically expressing Cdc24 or Cdc42 are unable to form hyphae in response to serum. Here we investigated the role of these two proteins in hyphal growth, using quantitative real-time PCR to measure induction of hypha-specific genes together with time lapse microscopy. Expression of the hypha-specific genes examined depends on the cyclic AMP-dependent protein kinase A pathway culminating in the Efg1 and Tec1 transcription factors. We show that strains with reduced levels of CDC24 or CDC42 transcripts induce hypha-specific genes yet cannot maintain their expression in response to serum. Furthermore, in serum these mutants form elongated buds compared to the wild type and mutant budding cells, as observed by time lapse microscopy. Using Cdc24 fused to green fluorescent protein, we also show that Cdc24 is recruited to and persists at the germ tube tip during hyphal growth. Altogether these data demonstrate that the Cdc24/Cdc42 GTPase module is required for maintenance of hyphal growth. In addition, overexpression studies indicate that specific levels of Cdc24 and Cdc42 are important for invasive hyphal growth. In response to serum, CDC24 transcript levels increase transiently in a Tec1-dependent fashion, as do the G-protein RHO3 and the Rho1 GTPase activating protein BEM2 transcript levels. These results suggest that a positive feedback loop between Cdc24 and Tec1 contributes to an increase in active Cdc42 at the tip of the germ tube which is important for hypha formation.
Figures

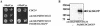
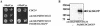
References
-
- Akashi, T., T. Kanbe, and K. Tanaka. 1994. The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology 140:271-280. - PubMed
-
- Anderson, J. M., and D. R. Soll. 1986. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J. Gen. Microbiol. 132:2035-2047. - PubMed
-
- Ayscough, K. R., and D. G. Drubin. 1998. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 8:927-930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

