Cellulase, clostridia, and ethanol
- PMID: 15755956
- PMCID: PMC1082790
- DOI: 10.1128/MMBR.69.1.124-154.2005
Cellulase, clostridia, and ethanol
Abstract
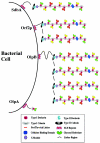
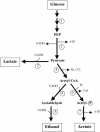
Biomass conversion to ethanol as a liquid fuel by the thermophilic and anaerobic clostridia offers a potential partial solution to the problem of the world's dependence on petroleum for energy. Coculture of a cellulolytic strain and a saccharolytic strain of Clostridium on agricultural resources, as well as on urban and industrial cellulosic wastes, is a promising approach to an alternate energy source from an economic viewpoint. This review discusses the need for such a process, the cellulases of clostridia, their presence in extracellular complexes or organelles (the cellulosomes), the binding of the cellulosomes to cellulose and to the cell surface, cellulase genetics, regulation of their synthesis, cocultures, ethanol tolerance, and metabolic pathway engineering for maximizing ethanol yield.
Figures




References
-
- Ahsan, M. M., M. Matsumoto, S. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 1997. Purification and characterization of the family J catalytic domain derived from the Clostridium thermocellum endoglucanase CelJ. Biosci. Biotechnol. Biochem. 61:427-431. - PubMed
-
- Ait, N., N. Creuzet, and J. Cattaneo. 1982. Properties of β-glucosidase purified from Clostridium thermocellum. J. Gen. Microbiol. 128:569-577.
-
- Ait, N., N. Creuzet, and P. Forget. 1979. Partial purification of cellulase from Clostridium thermocellum. J. Gen. Microbiol. 113:399-402.
-
- Alexander, J. K. 1968. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J. Biol. Chem. 243:2899-2904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

