Trends in UK cancer trials: results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials
- PMID: 15756251
- PMCID: PMC2361907
- DOI: 10.1038/sj.bjc.6602425
Trends in UK cancer trials: results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials
Abstract
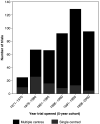
We aimed to study trends in the design and conduct of randomised controlled trials (RCTs) in cancer in the UK, using the UK Coordinating Committee for Cancer Research (UKCCCR) National Register of Cancer Trials (NRCT). We conducted a descriptive survey of 520 UK RCTs in cancer that were registered on the UKCCCR NRCT. All trials had been initiated between 1971 and 2000. Trials on the NRCT have been conducted in a wide variety of cancer types, but with a third in breast (22%) or lung cancer (11%). They have largely been funded by the UK public and charity sectors. Overall, there has been a sustained rise in the total numbers of patients entering UK cancer trials over time with a trend towards larger, multicentre trials, greater recruitment targets and a marked reduction in the average time taken to complete trials. Trends in the design and conduct of noncommercial cancer RCTs from 1971 to 2000 are encouraging. It will be interesting to see how they develop in light of the implementation of recent national initiatives regarding cancer clinical trials in the UK.
Figures





References
-
- Coombes R (2004) Body set up to increase numbers taking part in trials. BMJ 329: 70
-
- European Union Directive (2001) European Union Directive 2001/20/EC (94/04/2001). Off J Eur Community L121: 34–44
-
- Fayers PM (1995) The UKCCCR register of UK cancer trials. Clin Oncol 7: 72–76 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

