Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro
- PMID: 15757365
- PMCID: PMC1064849
- DOI: 10.1371/journal.pbio.0030087
Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro
Abstract
It has long been held as scientific fact that soon after birth, cardiomyocytes cease dividing, thus explaining the limited restoration of cardiac function after a heart attack. Recent demonstrations of cardiac myocyte differentiation observed in vitro or after in vivo transplantation of adult stem cells from blood, fat, skeletal muscle, or heart have challenged this view. Analysis of these studies has been complicated by the large disparity in the magnitude of effects seen by different groups and obscured by the recently appreciated process of in vivo stem-cell fusion. We now show a novel population of nonsatellite cells in adult murine skeletal muscle that progress under standard primary cell-culture conditions to autonomously beating cardiomyocytes. Their differentiation into beating cardiomyocytes is characterized here by video microscopy, confocal-detected calcium transients, electron microscopy, immunofluorescent cardiac-specific markers, and single-cell patch recordings of cardiac action potentials. Within 2 d after tail-vein injection of these marked cells into a mouse model of acute infarction, the marked cells are visible in the heart. By 6 d they begin to differentiate without fusing to recipient cardiac cells. Three months later, the tagged cells are visible as striated heart muscle restricted to the region of the cardiac infarct.
Figures


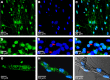

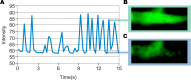



Comment in
-
Alchemy and the new age of cardiac muscle cell biology.PLoS Biol. 2005 Apr;3(4):e131. doi: 10.1371/journal.pbio.0030131. PLoS Biol. 2005. PMID: 15819607 Free PMC article.
References
-
- Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94:II332–II336. - PubMed
-
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: A hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. - PubMed
-
- Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: Characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27:924–933. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

