High mobility of flap endonuclease 1 and DNA polymerase eta associated with replication foci in mammalian S-phase nucleus
- PMID: 15758026
- PMCID: PMC1087254
- DOI: 10.1091/mbc.e04-12-1066
High mobility of flap endonuclease 1 and DNA polymerase eta associated with replication foci in mammalian S-phase nucleus
Abstract
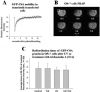
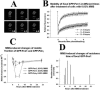
Originally detected in fixed cells, DNA replication foci (RFi) were later visualized in living cells by using green fluorescent protein (GFP)-tagged proliferating cell nuclear antigen (PCNA) and DNA ligase I. It was shown using fluorescence redistribution after photobleaching (FRAP) assay that focal GFP-PCNA slowly exchanged, suggesting the existence of a stable replication holocomplex. Here, we used the FRAP assay to study the dynamics of the GFP-tagged PCNA-binding proteins: Flap endonuclease 1 (Fen1) and DNA polymerase eta (Pol eta). We also used the GFP-Cockayne syndrome group A (CSA) protein, which does associate with transcription foci after DNA damage. In normal cells, GFP-Pol eta and GFP-Fen1 are mobile with residence times at RFi (t(m)) approximately 2 and approximately 0.8 s, respectively. GFP-CSA is also mobile but does not concentrate at discrete foci. After methyl methanesulfonate (MMS) damage, the mobile fraction of focal GFP-Fen1 decreased and t(m) increased, but it then recovered. The mobilities of focal GFP-Pol eta and GFP-PCNA did not change after MMS. The mobility of GFP-CSA did not change after UV-irradiation. These data indicate that the normal replication complex contains at least two mobile subunits. The decrease of the mobile fraction of focal GFP-Fen1 after DNA damage suggests that Fen1 exchange depends on the rate of movement of replication forks.
Figures



Similar articles
-
[Dynamics of some postreplication DNA repair proteins in carcinogen-damaged mammalian cells].Tsitologiia. 2004;46(1):43-52. Tsitologiia. 2004. PMID: 15112431 Russian.
-
Nuclear dynamics of PCNA in DNA replication and repair.Mol Cell Biol. 2005 Nov;25(21):9350-9. doi: 10.1128/MCB.25.21.9350-9359.2005. Mol Cell Biol. 2005. PMID: 16227586 Free PMC article.
-
Localisation of human Y-family DNA polymerase kappa: relationship to PCNA foci.J Cell Sci. 2005 Jan 1;118(Pt 1):129-36. doi: 10.1242/jcs.01603. Epub 2004 Dec 15. J Cell Sci. 2005. PMID: 15601657
-
Dynamics of mammalian NER proteins.DNA Repair (Amst). 2011 Jul 15;10(7):760-71. doi: 10.1016/j.dnarep.2011.04.015. Epub 2011 May 7. DNA Repair (Amst). 2011. PMID: 21550320 Review.
-
[Flap endonuclease-1 and its role in the processes of DNA metabolism in eucaryotic cells].Mol Biol (Mosk). 2008 May-Jun;42(3):405-21. Mol Biol (Mosk). 2008. PMID: 18702299 Review. Russian.
Cited by
-
Regulation of the Intranuclear Distribution of the Cockayne Syndrome Proteins.Sci Rep. 2018 Nov 30;8(1):17490. doi: 10.1038/s41598-018-36027-6. Sci Rep. 2018. PMID: 30504782 Free PMC article.
-
RAD18 and associated proteins are immobilized in nuclear foci in human cells entering S-phase with ultraviolet light-induced damage.Mutat Res. 2008 Dec 15;648(1-2):23-31. doi: 10.1016/j.mrfmmm.2008.09.006. Epub 2008 Sep 24. Mutat Res. 2008. PMID: 18926833 Free PMC article.
-
Replication-fork dynamics.Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1):a010157. doi: 10.1101/cshperspect.a010157. Cold Spring Harb Perspect Biol. 2014. PMID: 23881939 Free PMC article. Review.
-
Interaction of estrogen receptor alpha with proliferating cell nuclear antigen.Nucleic Acids Res. 2007;35(15):5028-38. doi: 10.1093/nar/gkm533. Epub 2007 Jul 18. Nucleic Acids Res. 2007. PMID: 17636311 Free PMC article.
-
Functional TFIIH is required for UV-induced translocation of CSA to the nuclear matrix.Mol Cell Biol. 2007 Apr;27(7):2538-47. doi: 10.1128/MCB.01288-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242193 Free PMC article.
References
-
- Avkin, S., and Livneh, Z. (2002). Efficiency, specificity and DNA polymerase-dependence of translesion replication across the oxidative DNA lesion 8-oxoguanine in human cells. Mutat. Res. 510, 81-90. - PubMed
-
- Cardoso, M. C., Leonhardt, H., and Nadal-Ginard, B. (1993). Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell 74, 979-992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

