Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures
- PMID: 15758028
- PMCID: PMC1087253
- DOI: 10.1091/mbc.e04-11-0968
Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures
Abstract
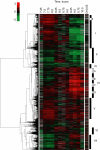
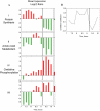
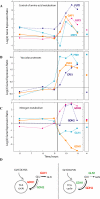
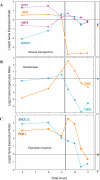
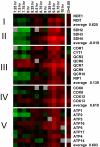
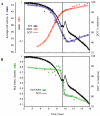
We studied the physiological response to glucose limitation in batch and steady-state (chemostat) cultures of Saccharomyces cerevisiae by following global patterns of gene expression. Glucose-limited batch cultures of yeast go through two sequential exponential growth phases, beginning with a largely fermentative phase, followed by an essentially completely aerobic use of residual glucose and evolved ethanol. Judging from the patterns of gene expression, the state of the cells growing at steady state in glucose-limited chemostats corresponds most closely with the state of cells in batch cultures just before they undergo this "diauxic shift." Essentially the same pattern was found between chemostats having a fivefold difference in steady-state growth rate (the lower rate approximating that of the second phase respiratory growth rate in batch cultures). Although in both cases the cells in the chemostat consumed most of the glucose, in neither case did they seem to be metabolizing it primarily through respiration. Although there was some indication of a modest oxidative stress response, the chemostat cultures did not exhibit the massive environmental stress response associated with starvation that also is observed, at least in part, during the diauxic shift in batch cultures. We conclude that despite the theoretical possibility of a switch to fully aerobic metabolism of glucose in the chemostat under conditions of glucose scarcity, homeostatic mechanisms are able to carry out metabolic adjustment as if fermentation of the glucose is the preferred option until the glucose is entirely depleted. These results suggest that some aspect of actual starvation, possibly a component of the stress response, may be required for triggering the metabolic remodeling associated with the diauxic shift.
Figures



References
-
- Alexander, M., and Jeffries, T. (1990). Respiratory efficiency and metabolite partitioning as regulatory phenomena in yeasts. Enzyme Microb. Technol. 12, 2-19.
-
- Boer, V. M., de Winde, J. H., Pronk, J. T., and Piper, M. D. (2003). The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278, 3265-3274. - PubMed
-
- Boy-Marcotte, E., Tadi, D., Perrot, M., Boucherie, H., and Jacquet, M. (1996). High cAMP levels antagonize the reprogramming of gene expression that occurs at the diauxic shift in Saccharomyces cerevisiae. Microbiology 142, 459-467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

