The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration
- PMID: 15758069
- PMCID: PMC556009
- DOI: 10.1073/pnas.0501160102
The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration
Abstract
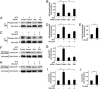
During the development of the peripheral nervous system, Schwann cells, the myelin-forming glia, migrate along axons before initiating myelination. We previously demonstrated that endogenous neurotrophin-3 (NT3) acting through the TrkC tyrosine kinase receptor enhances migration of premyelinating Schwann cells. This signaling pathway is mediated by the c-Jun N-terminal kinase (JNK) cascade regulated by the Rho GTPases Rac1 and Cdc42. However, missing is the link between TrkC and the GTPases. Here, we show that a guanine-nucleotide exchange factor (GEF), Dbl's big sister (Dbs), couples with TrkC to activate Cdc42 in Schwann cells. Furthermore, TrkC directly phosphorylates Dbs, thereby inducing the Cdc42-GEF activity. Taken together, activation of TrkC triggers Schwann cell migration by regulating Dbs upon direct tyrosine phosphorylation, providing a mechanism whereby a membrane receptor tyrosine kinase can induce the activation of Rho GTPase-GEFs.
Figures

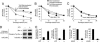


Comment in
-
Coupling neurotrophins to cell migration through selective guanine nucleotide exchange factor activation.Proc Natl Acad Sci U S A. 2005 Apr 19;102(16):5645-6. doi: 10.1073/pnas.0501718102. Epub 2005 Apr 12. Proc Natl Acad Sci U S A. 2005. PMID: 15827113 Free PMC article. No abstract available.
References
-
- Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. & Horwitz, A. R. (2003) Science 302, 1704–1709. - PubMed
-
- Bunge, R. P., Bunge, M. B. & Eldridge, C. F. (1986) Annu. Rev. Neurosci. 9, 305–328. - PubMed
-
- Huang, E. J. & Reichardt, L. F. (2003) Annu. Rev. Biochem. 72, 609–642. - PubMed
-
- Barker, P. A. (2004) Neuron 42, 529–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

