High-dose radiation with bone marrow transfer prevents neurodegeneration in an inherited glaucoma
- PMID: 15758074
- PMCID: PMC555465
- DOI: 10.1073/pnas.0407357102
High-dose radiation with bone marrow transfer prevents neurodegeneration in an inherited glaucoma
Abstract
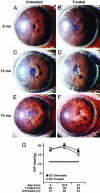
Here, we show that high-dose gamma-irradiation accompanied with syngeneic bone marrow transfer can confer complete protection against glaucoma in a mouse model. Because bone marrow genotype was unaltered by this procedure, it was not the causative agent. The neuroprotection is robust and highly reproducible. Glaucoma-prone DBA/2J mice received a single treatment at 5-8 weeks of age and were protected from glaucomatous retinal ganglion cell degeneration out to 14 months of age (oldest assessed). By 12-14 months, retinal ganglion cell degeneration is usually very severe and essentially complete in the majority of untreated DBA/2J mice. To assess reproducibility, three groups of mice were treated at different times, and the results were essentially the same each time. Considering all experiments, the vast majority of treated mice had no detectable glaucomatous neurodegeneration. A beneficial effect of treatment including high-dose radiation is unprecedented, and we are not aware of any other neuroprotective effects this substantial. Because of the robust protective effect, this treatment offers another tool for studying mechanisms of neuroprotection.
Figures


References
-
- Ritch, R., Shields, M. B. & Krupin, T. (1996) The Glaucomas (Mosby, St. Louis).
-
- Weinreb, R. N. & Khaw, P. T. (2004) Lancet 363, 1711–1720. - PubMed
-
- Heijl, A., Leske, M. C., Bengtsson, B., Hyman, L. & Hussein, M. (2002) Arch. Ophthalmol. 120, 1268–1279. - PubMed
-
- Collaborative Normal-Tension Glaucoma Study Group (1998) Am. J. Ophthalmol. 126, 487–497. - PubMed
-
- Wax, M. B. & Tezel, G. (2002) Mol. Neurobiol. 26, 45–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

