Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide
- PMID: 15758152
- PMCID: PMC3275418
- DOI: 10.1523/JNEUROSCI.4696-04.2005
Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide
Abstract
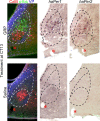
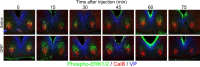
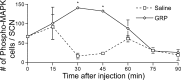
The circadian clock located in the mammalian suprachiasmatic nucleus (SCN) exhibits substantial heterogeneity in both its neurochemical and functional organization, with retinal input and oscillatory timekeeping functions segregated to different regions within the nucleus. Although it is clear that photic information must be relayed from directly retinorecipient cells to the population of oscillator cells within the nucleus, the intra-SCN signal (or signals) underlying such communication has yet to be identified. Gastrin-releasing peptide (GRP), which is found within calbindin-containing retinorecipient cells and causes photic-like phase shifts when applied directly to the SCN, is a candidate molecule. Here we examine the effect of GRP on both molecular and behavioral properties of the hamster circadian system. Within 30 min a third ventricle injection of GRP produces an increase in the number of cells expressing the phosphorylated form of extracellular signal-regulated kinases 1/2 (p-ERK1/2), localized in a discrete group of SCN cells that form a cap dorsal to calbindin cells and lateral to vasopressin cells. At 1 h after the peak of p-ERK expression these cap cells express c-fos, Period1, and Period2. Pharmacological blockade of ERK phosphorylation attenuates phase shifts to GRP. These data indicate that GRP is an output signal of retinorecipient SCN cells and activates a small cluster of SCN neurons. This novel cell group likely serves as a relay or integration point for communicating photic phase-resetting information to the rhythmic cells of the SCN. These findings represent a first step in deconstructing the SCN network constituting the brain clock.
Figures




References
-
- Abe H, Rusak B (1994) Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev 18: 531-536. - PubMed
-
- Abe H, Rusak B, Robertson HA (1991) Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett 127: 9-12. - PubMed
-
- Abe H, Rusak B, Robertson HA (1992) NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull 28: 831-835. - PubMed
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S (2002) Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61: 26-34. - PubMed
-
- Antle MC, Silver R (2005) Orchestrating time: arrangements of the brain's circadian clock. Trends Neurosci, in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
