Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation
- PMID: 15758153
- PMCID: PMC6725159
- DOI: 10.1523/JNEUROSCI.4391-04.2005
Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation
Erratum in
- J Neurosci. 2005 May 4;25(18):4658
Abstract
Recent studies support the hypothesis that soluble oligomers of amyloid beta-peptide (Abeta) rather than mature amyloid fibrils are the earliest effectors of synaptic compromise in Alzheimer's disease. We took advantage of an amyloid precursor protein-overexpressing cell line that secretes SDS-stable Abeta oligomers to search for inhibitors of the pathobiological effects of natural human Abeta oligomers. Here, we identify small molecules that inhibit formation of soluble Abeta oligomers and thus abrogate their block of long-term potentiation (LTP). Furthermore, we show that cell-derived Abeta oligomers can be separated from monomers by size exclusion chromatography under nondenaturing conditions and that the isolated, soluble oligomers, but not monomers, block LTP. The identification of small molecules that inhibit early Abeta oligomer formation and rescue LTP inhibition offers a rational approach for therapeutic intervention in Alzheimer's disease and highlights the utility of our cell-culture paradigm as a useful secondary screen for compounds designed to inhibit early steps in Abeta oligomerization under biologically relevant conditions.
Figures
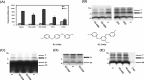
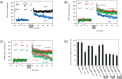

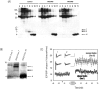
References
-
- Cohen FE, Kelly JW (2003) Therapeutic approaches to protein-misfolding diseases. Nature 426: 905-909. - PubMed
-
- Davies CA, Mann DM, Sumpter PQ, Yates PO (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci 78: 151-164. - PubMed
-
- Findeis MA (2002) Peptide inhibitors of beta amyloid aggregation. Curr Top Med Chem 2: 417-423. - PubMed
-
- Haass C, Schlossmacher M, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski B, Lieberburg I, Koo EH, Schenk D, Teplow D, Selkoe D (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322-325. - PubMed
-
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
