Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS
- PMID: 15758163
- PMCID: PMC6725171
- DOI: 10.1523/JNEUROSCI.4794-04.2005
Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS
Abstract
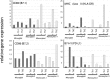
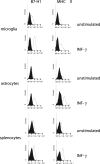
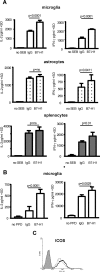
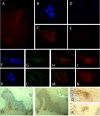
Inflammation of the CNS is usually locally limited to avoid devastating consequences. Critical players involved in this immune regulatory process are the resident immune cells of the brain, the microglia. Interactions between the growing family of B7 costimulatory ligands and their receptors are increasingly recognized as important pathways for costimulation and/or inhibition of immune responses. Human and mouse microglial cells constitutively express B7 homolog 1 (B7-H1) in vitro. However, under inflammatory conditions [presence of interferon-gamma (IFN-gamma) or T-helper 1 supernatants], a significant upregulation of B7-H1 was detectable. Expression levels of B7-H1 protein on microglial cells were substantially higher compared with astrocytes or splenocytes. Coculture experiments of major histocompatibility complex class II-positive antigen-presenting cells (APC) with syngeneic T cells in the presence of antigen demonstrated the functional consequences of B7-H1 expression on T-cell activation. In the presence of a neutralizing anti-B7-H1 antibody, both the production of inflammatory cytokines (IFN-gamma and interleukin-2) and the upregulation of activation markers (inducible costimulatory signal) by T cells were markedly enhanced. Interestingly, this effect was clearly more pronounced when microglial cells were used as APC, compared with astrocytes or splenocytes. Furthermore, B7-H1 was highly upregulated during the course of myelin oligodendrocyte glycoprotein-induced and proteolipid protein-induced experimental allergic encephalomyelitis in vivo. Expression was predominantly localized to areas of strongest inflammation and could be colocalized with microglial cells/macrophages as well as T cells. Together, our data propose microglial B7-H1 as an important immune inhibitory molecule capable of downregulating T-cell activation in the CNS and thus confining immunopathological damage.
Figures



References
-
- Aloisi F (2001) Immune function of microglia. Glia 36: 165-179. - PubMed
-
- Aloisi F, Penna G, Polazzi E, Minghetti L, Adorini L (1999) CD40-CD154 interaction and IFN-gamma are required for IL-12 but not prosta-glandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol 162: 1384-1391. - PubMed
-
- Barker CF, Billingham RE (1977) Immunologically privileged sites. Adv Immunol 25: 1-54. - PubMed
-
- Becher B, Antel JP (1996) Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18: 1-10. - PubMed
-
- Becher B, Prat A, Antel JP (2000) Brain-immune connection: immunoregulatory properties of CNS-resident cells. Glia 29: 293-304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
