Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons
- PMID: 15758167
- PMCID: PMC2605385
- DOI: 10.1523/JNEUROSCI.4118-04.2005
Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons
Abstract
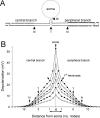
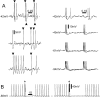
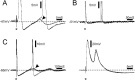
Ectopic discharge generated in injured afferent axons and cell somata in vivo contributes significantly to chronic neuropathic dysesthesia and pain after nerve trauma. Progress has been made toward understanding the processes responsible for this discharge using a preparation consisting of whole excised dorsal root ganglia (DRGs) with the cut nerve attached. In the in vitro preparation, however, spike activity originates in the DRG cell soma but rarely in the axon. We have now overcome this impediment to understanding the overall electrogenic processes in soma and axon, including the resulting discharge patterns, by modifying the bath medium in which recordings are made. At both sites, bursts can be triggered by subthreshold oscillations, a phasic stimulus, or spikes arising elsewhere in the neuron. In the soma, once triggered, bursts are maintained by depolarizing afterpotentials, whereas in the axon, an additional process also plays a role, delayed depolarizing potentials. This alternative process appears to be involved in "clock-like" bursting, a discharge pattern much more common in axons than somata. Ectopic spikes arise alternatively in the soma, the injured axon end (neuroma), and the region of the axonal T-junction. Discharge sequences, and even individual multiplet bursts, may be a mosaic of action potentials that originate at these alternative electrogenic sites within the neuron. Correspondingly, discharge generated at these alternative sites may interact, explaining the sometimes-complex firing patterns observed in vivo.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
