Physiologic variability of single-voxel proton MR spectroscopic measurements at 3T
- PMID: 15760870
- PMCID: PMC7976485
Physiologic variability of single-voxel proton MR spectroscopic measurements at 3T
Abstract
Background and purpose: Physiologic and scanner variability of proton MR spectroscopy (MRS) measurements can limit the detection of subtle metabolite fluctuations. We assessed the variability of such measurements at 3T and compared two methods to obtain absolute concentrations.
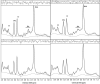
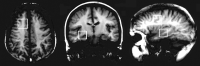
Methods: Variability over 14 days was assessed with short-echo, single-voxel proton MRS in 14 control subjects and in a phantom containing 50 mmol/L N-acetylaspartate (NAA). Spectra were analyzed by using LCModel, scaling factors determined with both the calibration phantom (CP), and water peak intensity (WP) methods. Relative (reflecting the systematic drift) and absolute variability (reflecting the magnitude of scanner variability) was determined.
Results: For the phantom, initial (49 +/- 1.7 mmol/L) and second measurements (50 +/- 1.6 mmol/L) showed similar results, with small variability (relative, -0.6 +/- 1.5 mmol/L; absolute, 1.1 +/- 1.1 mmol/L). Control subjects had no systematic difference between the two scans for any measurement. Absolute variabilities in the temporal lobe for total NAA (NAA+NAAG) were 13% (CP) and 11% (WP). The largest variability (29%) was found for glutamate-glutamine (29%) with the CP method, and for myo-inositol with the WP method (28%). Absolute variability was smaller for the frontal lobe measurements (total NAA 7% and overall 6-18% for CP; total NAA 6% and overall 5-19% for WP). No significant difference was observed between the two methods.
Conclusion: Physiologic variability is the major source of measurement variability and accounts for 12% of the variability in temporal lobe total NAA. Therefore, total NAA variations must clearly exceed this before they can reliably be attributed to an effect of disease.
Figures
Similar articles
-
Validation of in vivo MRS measures of metabolite concentrations in the human brain.NMR Biomed. 2019 Mar;32(3):e4058. doi: 10.1002/nbm.4058. Epub 2019 Jan 21. NMR Biomed. 2019. PMID: 30663818
-
MR-spectroscopy in metachromatic leukodystrophy: A model free approach and clinical correlation.Neuroimage Clin. 2023;37:103296. doi: 10.1016/j.nicl.2022.103296. Epub 2022 Dec 20. Neuroimage Clin. 2023. PMID: 36563646 Free PMC article.
-
Reproducibility and reliability of short-TE whole-brain MR spectroscopic imaging of human brain at 3T.Magn Reson Med. 2015 Mar;73(3):921-8. doi: 10.1002/mrm.25208. Epub 2014 Mar 26. Magn Reson Med. 2015. PMID: 24677384 Free PMC article.
-
A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson's disease.Dement Geriatr Cogn Disord. 2002;14(2):64-76. doi: 10.1159/000064927. Dement Geriatr Cogn Disord. 2002. PMID: 12145453 Review.
-
Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): a review.Autism Res. 2013 Apr;6(2):119-33. doi: 10.1002/aur.1273. Epub 2013 Feb 21. Autism Res. 2013. PMID: 23436782 Review.
Cited by
-
Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise.Bone. 2014 Jul;64:39-46. doi: 10.1016/j.bone.2014.03.044. Epub 2014 Apr 5. Bone. 2014. PMID: 24709686 Free PMC article.
-
Longitudinal inter- and intra-individual human brain metabolic quantification over 3 years with proton MR spectroscopy at 3 T.Magn Reson Med. 2012 Jan;67(1):27-33. doi: 10.1002/mrm.23001. Epub 2011 Jun 7. Magn Reson Med. 2012. PMID: 21656555 Free PMC article.
-
Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment.Sleep. 2012 Jan 1;35(1):41-8. doi: 10.5665/sleep.1582. Sleep. 2012. PMID: 22215917 Free PMC article.
-
Reproducibility of quantitative structural and physiological MRI measurements.Brain Behav. 2017 Aug 2;7(9):e00759. doi: 10.1002/brb3.759. eCollection 2017 Sep. Brain Behav. 2017. PMID: 28948069 Free PMC article.
-
Recent advances in magnetic resonance neurospectroscopy.Neurotherapeutics. 2007 Jul;4(3):330-45. doi: 10.1016/j.nurt.2007.04.009. Neurotherapeutics. 2007. PMID: 17599700 Free PMC article. Review.
References
-
- Kuzniecky R. Magnetic resonance spectroscopy in focal epilepsy: 31P and 1H spectroscopy. Rev Neurol (Paris) 1999;155:495–498 - PubMed
-
- Kuzniecky R, Hugg JW, Hetherington H. Relative utility of 1H spectroscopic imaging and hippocampal volumetry in the lateralization of mesial temporal lobe epilepsy. Neurology 1998;51:66–71 - PubMed
-
- Kikuchi S, Kubota F, Akata T, et al. A study of the relationship between the seizure focus and 1H-MRS in temporal lobe epilepsy and frontal lobe epilepsy. Psychiatry Clin Neurosci 2000;54:455–459 - PubMed
-
- Li LM, Cendes F, Antel SB, et al. Prognostic value of proton magnetic resonance spectroscopic imaging for surgical outcome in patients with intractable temporal lobe epilepsy and bilateral hippocampal atrophy. Ann Neurol 2000;47:195–200 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
