Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones
- PMID: 15760934
- PMCID: PMC1464485
- DOI: 10.1113/jphysiol.2005.084871
Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones
Abstract
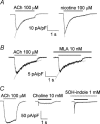
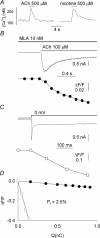
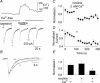
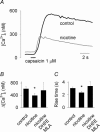
Ca2+ entry through neuronal nicotinic ACh receptors (nAChRs) modulates many biological processes in nervous tissue. In order to study the functional role of nAChRs in peripheral sensory signalling, we measured their Ca2+ permeability in rat dorsal root ganglion (DRG) neurones, and analysed the effects of nAChR-mediated Ca2+ influx on the function of the vanilloid receptor TRPV1. The fractional Ca2+ current (P(f), i.e. the percentage of current carried by Ca2+ ions) flowing through nAChR channels was measured by Ca2+ imaging fluorescence microscopy in combination with the patch-clamp technique. Functional nAChRs were expressed in a subset of adult DRG neurones (about 24% of the cells), typically with small to medium size as measured by their capacitance (40 +/- 3 pF). In most cells, ACh evoked slowly desensitizing currents, insensitive to methyllycaconitine (MLA, 10 nm), a potent antagonist of homomeric nAChRs. Fast decaying currents, probably mediated by alpha7*-nAChRs (i.e. native alpha7-containing nAChRs), were observed in 15% of ACh-responsive cells, in which slowly decaying currents, mediated by heteromeric nAChRs, were simultaneously present. The nAChRs of adult DRG neurones exhibited a P(f) value of 2.2 +/- 0.6% in the presence of MLA and 1.9 +/- 0.6% (P > 0.1) in the absence of MLA, indicating that homomeric MLA-sensitive nAChRs do not contribute to Ca2+ entry into adult DRG neurones. Conversely, 10% of neonatal DRG neurones showed ACh-evoked currents completely blocked by MLA. In these neurones, nAChRs showed a larger P(f) value (9.5 +/- 1.5%), indicating the expression of bona fide alpha7*-nAChRs. Finally, we report that Ca2+ influx through nAChRs in adult DRG neurones negatively modulated the TRPV1-mediated responses, representing a possible mechanism underlying the analgesic properties of nicotinic agonists on sensory neurones.
Figures

Similar articles
-
Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors.Cell Calcium. 2006 Apr;39(4):349-55. doi: 10.1016/j.ceca.2005.12.002. Epub 2006 Jan 31. Cell Calcium. 2006. PMID: 16451809
-
Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG.J Neurophysiol. 2005 Mar;93(3):1358-71. doi: 10.1152/jn.00591.2004. Epub 2004 Oct 13. J Neurophysiol. 2005. PMID: 15483069
-
Functional contribution of the alpha7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones.J Physiol. 1998 Jun 15;509 ( Pt 3)(Pt 3):651-65. doi: 10.1111/j.1469-7793.1998.651bm.x. J Physiol. 1998. PMID: 9596789 Free PMC article.
-
Nicotinic acetylcholine receptors in autonomic ganglia.Auton Neurosci. 2002 Apr 18;97(1):1-11. doi: 10.1016/s1566-0702(01)00386-1. Auton Neurosci. 2002. PMID: 12036180 Review.
-
Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system.Acta Pharmacol Sin. 2009 Jun;30(6):673-80. doi: 10.1038/aps.2009.64. Epub 2009 May 18. Acta Pharmacol Sin. 2009. PMID: 19448647 Free PMC article. Review.
Cited by
-
Functional α6β4 acetylcholine receptor expression enables pharmacological testing of nicotinic agonists with analgesic properties.J Clin Invest. 2020 Nov 2;130(11):6158-6170. doi: 10.1172/JCI140311. J Clin Invest. 2020. PMID: 33074244 Free PMC article.
-
Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models.Neuropharmacology. 2013 Feb;65:156-64. doi: 10.1016/j.neuropharm.2012.08.022. Epub 2012 Oct 16. Neuropharmacology. 2013. PMID: 23079470 Free PMC article.
-
{alpha}7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis.Am J Physiol Gastrointest Liver Physiol. 2010 Sep;299(3):G761-8. doi: 10.1152/ajpgi.00175.2010. Epub 2010 Jul 1. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20595621 Free PMC article.
-
Gut sensory neurons as regulators of neuro-immune-microbial interactions: from molecular mechanisms to precision therapy for IBD/IBS.J Neuroinflammation. 2025 Jul 2;22(1):172. doi: 10.1186/s12974-025-03500-9. J Neuroinflammation. 2025. PMID: 40605050 Free PMC article. Review.
-
Role of nicotinic receptors and acetylcholine in mucous cell metaplasia, hyperplasia, and airway mucus formation in vitro and in vivo.J Allergy Clin Immunol. 2012 Sep;130(3):770-780.e11. doi: 10.1016/j.jaci.2012.04.002. Epub 2012 May 9. J Allergy Clin Immunol. 2012. PMID: 22578901 Free PMC article.
References
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;12:2734–2742. - PubMed
-
- Brioni JD, Decker MW, Sullivan JP, Arneric SP. The pharmacology of (–)-nicotine and novel cholinergic channel modulators. Adv Pharmacol. 1997;37:153–214. - PubMed
-
- Caggiula AR, Epstein LH, Perkins KA, Saylor S. Different methods of assessing nicotine-induced antinociception may engage different neural mechanisms. Psychopharmacology. 1995;122:301–306. - PubMed
-
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

