Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones
- PMID: 15760941
- PMCID: PMC1464456
- DOI: 10.1113/jphysiol.2005.083089
Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones
Abstract
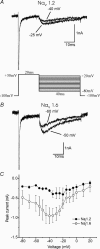
Sodium channels Na(v)1.2 and Na(v)1.6 are both normally expressed along premyelinated and myelinated axons at different stages of maturation and are also expressed in a subset of demyelinated axons, where coexpression of Na(v)1.6 together with the Na(+)/Ca(2+) exchanger is associated with axonal injury. It has been difficult to distinguish the currents produced by Na(v)1.2 and Na(v)1.6 in native neurones, and previous studies have not compared these channels within neuronal expression systems. In this study, we have characterized and directly compared Na(v)1.2 and Na(v)1.6 in a mammalian neuronal cell background and demonstrate differences in their properties that may affect neuronal behaviour. The Na(v)1.2 channel displays more depolarized activation and availability properties that may permit conduction of action potentials, even with depolarization. However, Na(v)1.2 channels show a greater accumulation of inactivation at higher frequencies of stimulation (20-100 Hz) than Na(v)1.6 and thus are likely to generate lower frequencies of firing. Na(v)1.6 channels produce a larger persistent current that may play a role in triggering reverse Na(+)/Ca(2+) exchange, which can injure demyelinated axons where Na(v)1.6 and the Na(+)/Ca(2+) exchanger are colocalized, while selective expression of Na(v)1.2 may support action potential electrogenesis, at least at lower frequencies, while producing a smaller persistent current.
Figures



References
-
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophysiol. 1997;77:1503–1513. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Mol Brain Res. 1996;43:117–131. - PubMed
-
- Black JA, Renganathan M, Waxman SG. Sodium channel Nav1.6 is expressed along nonmyelinated axons and it contributes to conduction. Mol Brain Res. 2002;105:19–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

