Effect of the oral endothelin antagonist bosentan on the clinical, exercise, and haemodynamic status of patients with pulmonary arterial hypertension related to congenital heart disease
- PMID: 15761050
- PMCID: PMC1769173
- DOI: 10.1136/hrt.2004.051961
Effect of the oral endothelin antagonist bosentan on the clinical, exercise, and haemodynamic status of patients with pulmonary arterial hypertension related to congenital heart disease
Abstract
Objective: To evaluate the clinical, exercise, and haemodynamic effects of chronic oral administration of the non-selective endothelin receptor antagonist bosentan on patients with pulmonary arterial hypertension (PAH) related to congenital heart disease (CHD).
Design: Prospective non-randomised open clinical study.
Setting: Cardiology tertiary referral centre.
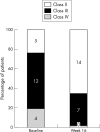
Patients: 21 patients with a mean (SEM) age of 22 (3) years with chronic PAH related to CHD (15 with Eisenmenger's syndrome). Patients were in World Health Organization (WHO) class II to IV with oxygen saturation 87 (2)%.
Intervention: Patients underwent clinical, exercise, and haemodynamic evaluations at baseline and after 16 weeks of treatment.
Results: Bosentan improved (p < 0.01) WHO class, peak oxygen consumption from 16.8 (1.4) to 18.3 (1.4) ml/kg/min, exercise duration from 9.0 (0.8) to 10.7 (0.6) minutes during the treadmill test, walking distance from 416 (23) to 459 (22) m, and Borg dyspnoea index from 2.8 (0.2) to 2.0 (0.1) during the six minute walk test. Bosentan treatment improved (p < 0.05) mean pulmonary artery pressure from 87 (4) to 81 (4) mm Hg, pulmonary blood flow index from 3.2 (0.4) to 3.7 (0.5) l/min/m2, pulmonary to systemic blood flow ratio from 1.2 (0.2) to 1.4 (0.2), and pulmonary vascular resistance index from 2232 (283) to 1768 (248) dyn.s.cm(-5). Two patients died, presumably of arrhythmic causes, who were in WHO class IV at baseline and who had improved during treatment.
Conclusions: Bosentan induces short and mid term clinical, exercise, and haemodynamic improvements in patients with PAH related to CHD. Larger studies with long term endothelin receptor antagonism are needed to assess the safety and possible treatment role of bosentan in this population.
Figures

References
-
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336:111–7. - PubMed
-
- MacGregor AJ, Canavan R, Knight C, et al. Pulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survival. Rheumatology (Oxford) 2001;40:453–9. - PubMed
-
- Ivy D. Diagnosis and treatment of severe pediatric pulmonary hypertension. Cardiol Rev 2001;9:227–37. - PubMed
-
- Daliento L, Somerville J, Presbitero P, et al. Eisenmenger syndrome: factors relating to deterioration and death. Eur Heart J 1998;19:1845–55. - PubMed
-
- Wagenvoort CA, Wagenvoort N. Pathology of the Eisenmenger syndrome and primary pulmonary hypertension. Adv Cardiol 1974;11:123–30. - PubMed
