Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion
- PMID: 15761191
- PMCID: PMC3229922
- DOI: 10.1161/01.ATV.0000161928.16334.dd
Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion
Abstract
Objective: Pharmacological inhibition of the cholesteryl ester transfer protein (CETP) in humans increases high-density lipoprotein (HDL) cholesterol (HDL-C) levels; however, its effects on apolipoprotein A-I (apoA-I) containing HDL subspecies, apoA-I turnover, and markers of reverse cholesterol transport are unknown. The present study was designed to address these issues.
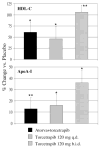
Methods and results: Nineteen subjects, 9 of whom were taking 20 mg of atorvastatin for hypercholesterolemia, received placebo for 4 weeks, followed by the CETP inhibitor torcetrapib (120 mg QD) for 4 weeks. In 6 subjects from the nonatorvastatin cohort, the everyday regimen was followed by a 4-week period of torcetrapib (120 mg BID). At the end of each phase, subjects underwent a primed-constant infusion of (5,5,5-2H3)-L-leucine to determine the kinetics of HDL apoA-I. The lipid data in this study have been reported previously. Relative to placebo, 120 mg daily torcetrapib increased the amount of apoA-I in alpha1-migrating HDL in the atorvastatin (136%; P<0.001) and nonatorvastatin (153%; P<0.01) cohorts, whereas an increase of 382% (P<0.01) was observed in the 120 mg twice daily group. HDL apoA-I pool size increased by 8+/-15% in the atorvastatin cohort (P=0.16) and by 16+/-7% (P<0.0001) and 34+/-8% (P<0.0001) in the nonatorvastatin 120 mg QD and BID cohorts, respectively. These changes were attributable to reductions in HDL apoA-I fractional catabolic rate (FCR), with torcetrapib reducing HDL apoA-I FCR by 7% (P=0.10) in the atorvastatin cohort, by 8% (P<0.001) in the nonatorvastatin 120 mg QD cohort, and by 21% (P<0.01) in the nonatorvastatin 120 mg BID cohort. Torcetrapib did not affect HDL apoA-I production rate. In addition, torcetrapib did not significantly change serum markers of cholesterol or bile acid synthesis or fecal sterol excretion.
Conclusions: These data indicate that partial inhibition of CETP via torcetrapib in patients with low HDL-C: (1) normalizes apoA-I levels within alpha1-migrating HDL, (2) increases plasma concentrations of HDL apoA-I by delaying apoA-I catabolism, and (3) does not significantly influence fecal sterol excretion.
Figures
References
-
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. - PubMed
-
- Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. - PubMed
-
- Miller NE. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J. 1987;113:589–597. - PubMed
-
- Manninen V, Elo MO, Frick MH, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. J Am Med Assoc. 1988;260:641–651. - PubMed
-
- Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. J Am Med Assoc. 2001;285:1585–1591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

