Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1
- PMID: 15761499
- PMCID: PMC1062893
- DOI: 10.1172/JCI23962
Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1
Erratum in
- J Clin Invest. 2005 Aug;115(8):2297
Abstract
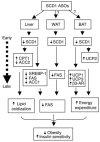
Effective therapies for the treatment of obesity, a key element of metabolic syndrome, are urgently needed but currently lacking. Stearoyl-CoA desaturase-1 (SCD1) is the rate-limiting enzyme catalyzing the conversion of saturated long-chain fatty acids into monounsaturated fatty acids, which are major components of triglycerides. In the current study, we tested the efficacy of pharmacological inhibition of SCD1 in controlling lipogenesis and body weight in mice. SCD1-specific antisense oligonucleotide inhibitors (ASOs) reduced SCD1 expression, reduced fatty acid synthesis and secretion, and increased fatty acid oxidization in primary mouse hepatocytes. Treatment of mice with SCD1 ASOs resulted in prevention of diet-induced obesity with concomitant reductions in SCD1 expression and the ratio of oleate to stearoyl-CoA in tissues and plasma. These changes correlated with reduced body adiposity, hepatomegaly and steatosis, and postprandial plasma insulin and glucose levels. Furthermore, SCD1 ASOs reduced de novo fatty acid synthesis, decreased expression of lipogenic genes, and increased expression of genes promoting energy expenditure in liver and adipose tissues. Thus, SCD1 inhibition represents a new target for the treatment of obesity and related metabolic disorders.
Figures
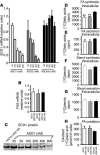
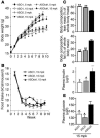
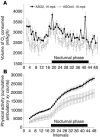

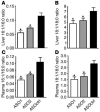
Comment in
-
Probing the role of stearoyl-CoA desaturase-1 in hepatic insulin resistance.J Clin Invest. 2006 Jun;116(6):1478-81. doi: 10.1172/JCI28774. J Clin Invest. 2006. PMID: 16741573 Free PMC article.
References
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. - PubMed
-
- Abelson P, Kennedy D. The obesity epidemic. Science. 2004;304:1413. - PubMed
-
- Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 1976;251:5095–5103. - PubMed
-
- Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004;43:91–104. - PubMed
-
- Zheng Y, et al. Scd3--a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

