Spectroscopy of non-heme iron thiolate complexes: insight into the electronic structure of the low-spin active site of nitrile hydratase
- PMID: 15762709
- PMCID: PMC4489710
- DOI: 10.1021/ic0487068
Spectroscopy of non-heme iron thiolate complexes: insight into the electronic structure of the low-spin active site of nitrile hydratase
Abstract
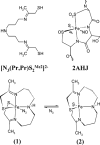
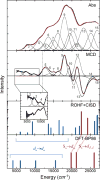
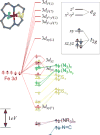
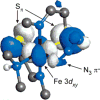
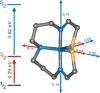
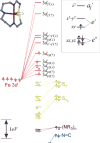
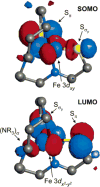
Detailed spectroscopic and computational studies of the low-spin iron complexes [Fe(III)(S2(Me2)N3 (Pr,Pr))(N3)] (1) and [Fe(III)(S2(Me2)N3 (Pr,Pr))]1+ (2) were performed to investigate the unique electronic features of these species and their relation to the low-spin ferric active sites of nitrile hydratases. Low-temperature UV/vis/NIR and MCD spectra of 1 and 2 reflect electronic structures that are dominated by antibonding interactions of the Fe 3d manifold and the equatorial thiolate S 3p orbitals. The six-coordinate complex 1 exhibits a low-energy S(pi) --> Fe 3d(xy) (approximately 13,000 cm(-1)) charge-transfer transition that results predominantly from the low energy of the singly occupied Fe 3d(xy) orbital, due to pure pi interactions between this acceptor orbital and both thiolate donor ligands in the equatorial plane. The 3d(pi) --> 3d(sigma) ligand-field transitions in this species occur at higher energies (>15,000 cm(-1)), reflecting its near-octahedral symmetry. The Fe 3d(xz,yz) --> Fe 3d(xy) (d(pi) --> d(pi)) transition occurs in the near-IR and probes the Fe(III)-S pi-donor bond; this transition reveals vibronic structure that reflects the strength of this bond (nu(e) approximately 340 cm(-1)). In contrast, the ligand-field transitions of the five-coordinate complex 2 are generally at low energy, and the S(pi) --> Fe charge-transfer transitions occur at much higher energies relative to those in 1. This reflects changes in thiolate bonding in the equatorial plane involving the Fe 3d(xy) and Fe 3d(x2-y2) orbitals. The spectroscopic data lead to a simple bonding model that focuses on the sigma and pi interactions between the ferric ion and the equatorial thiolate ligands, which depend on the S-Fe-S bond angle in each of the complexes. These electronic descriptions provide insight into the unusual S = 1/2 ground spin state of these complexes: the orientation of the thiolate ligands in these complexes restricts their pi-donor interactions to the equatorial plane and enforces a low-spin state. These anisotropic orbital considerations provide some intriguing insights into the possible electronic interactions at the active site of nitrile hydratases and form the foundation for further studies into these low-spin ferric enzymes.
Figures




References
-
- Holm RH, Kennepohl P, Solomon EI. Chem Rev. 1996;96:2239–2314. - PubMed
-
- Stephens PJ, Jollie DR, Warshel A. Chem Rev. 1996;96:2491–2513. - PubMed
-
- Beinert H. J Biol Inorg Chem. 2000;5:2–15. - PubMed
-
- Costas M, Mehn MP, Jensen MP, Que L. Chem Rev. 2004;104:939–986. - PubMed
-
- Que L, Ho RYN. Chem Rev. 1996;96:2607–2624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

