Evidence for involvement of peptidoglycan in the triggering of an oxidative burst by Listeria monocytogenes in phagocytes
- PMID: 15762877
- PMCID: PMC1809335
- DOI: 10.1111/j.1365-2249.2005.02740.x
Evidence for involvement of peptidoglycan in the triggering of an oxidative burst by Listeria monocytogenes in phagocytes
Abstract
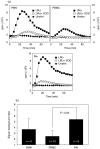
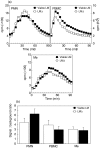
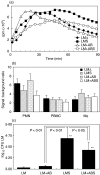
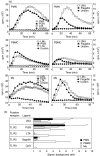
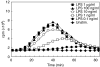
We have shown previously that in listeric encephalitis of cattle and rats, nitrotyrosine was produced in microabscesses, implying that both superoxide anion (O(2) (-)) and nitric oxide (NO) are present and react with each other. Evidence of local synthesis of NO by macrophages was provided, but the source of O(2) (-) remained unknown. Here we have examined whether phagocytes exposed to viable and heat-killed Listeria monocytogenes (LMDelta) produce O(2) (-) and, if so, whether this results from direct interaction of phagocytes with the bacterial surface of L. monocytogenes or whether prior opsonization is required. Using lucigenin-enhanced chemiluminescence (LCL) for the measurement of O(2) (-), we show that LMDelta induces an oxidative burst in human neutrophils, monocytes and monocyte-derived macrophages (Mphi). Viability is not required, and opsonization by antibodies and/or complement does not enhance the LCL signal. As Toll-like receptors (TLR) were shown recently to mediate an oxidative burst, TLR agonists representative for pathogen-associated molecular patterns (PAMPs) were tested for their ability to elicit an oxidative burst. These included lipoteichoic acid (LTA), bacterial peptidoglycan (PGN), recombinant flagellin, CpG-containing DNA and double-stranded RNA. Only PGN and flagellin consistently elicited an LCL signal resembling that induced by LMDelta with regard to the kinetics and cell spectrum stimulated. However, flagellin was unlikely to be responsible for the LMDelta-mediated burst, as a flagellin-deficient mutant showed no decrease in LCL. We therefore assume that in LMDelta, core PGN acts as a PAMP and directly induces an oxidative burst in all phagocyte populations. We conclude that in cerebral lesions superoxide anion is generated locally by phagocytes recognizing bacterial PGN.
Figures
Similar articles
-
Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils.Immunol Lett. 2003 Jan 2;85(1):75-80. doi: 10.1016/s0165-2478(02)00210-9. Immunol Lett. 2003. PMID: 12505201
-
Oxidative burst mediated by toll like receptors (TLR) and CD14 on avian heterophils stimulated with bacterial toll agonists.Dev Comp Immunol. 2003 May;27(5):423-9. doi: 10.1016/s0145-305x(02)00115-5. Dev Comp Immunol. 2003. PMID: 12631524
-
Differential induction of nitric oxide, degranulation, and oxidative burst activities in response to microbial agonist stimulations in monocytes and heterophils from young commercial turkeys.Vet Immunol Immunopathol. 2008 Jun 15;123(3-4):177-85. doi: 10.1016/j.vetimm.2008.01.033. Epub 2008 Jan 21. Vet Immunol Immunopathol. 2008. PMID: 18304649
-
Charge compensation during the phagocyte respiratory burst.Biochim Biophys Acta. 2006 Aug;1757(8):996-1011. doi: 10.1016/j.bbabio.2006.01.005. Epub 2006 Jan 30. Biochim Biophys Acta. 2006. PMID: 16483534 Review.
-
Phagocytic leukocytes and reactive oxygen species.Histochem Cell Biol. 2009 Apr;131(4):465-9. doi: 10.1007/s00418-009-0565-5. Epub 2009 Feb 18. Histochem Cell Biol. 2009. PMID: 19224236 Review.
Cited by
-
Listeriolysin O suppresses phospholipase C-mediated activation of the microbicidal NADPH oxidase to promote Listeria monocytogenes infection.Cell Host Microbe. 2011 Dec 15;10(6):627-34. doi: 10.1016/j.chom.2011.11.005. Cell Host Microbe. 2011. PMID: 22177565 Free PMC article.
-
TLR signalling augments macrophage bactericidal activity through mitochondrial ROS.Nature. 2011 Apr 28;472(7344):476-80. doi: 10.1038/nature09973. Nature. 2011. PMID: 21525932 Free PMC article.
-
Peptoanaerobacter stomatis Primes Human Neutrophils and Induces Granule Exocytosis.Infect Immun. 2017 Jun 20;85(7):e01043-16. doi: 10.1128/IAI.01043-16. Print 2017 Jul. Infect Immun. 2017. PMID: 28438978 Free PMC article.
-
Basic Analysis of the Cerebrospinal Fluid: An Important Framework for Laboratory Diagnostics of the Impairment of the Central Nervous System.Curr Issues Mol Biol. 2022 Aug 14;44(8):3666-3680. doi: 10.3390/cimb44080251. Curr Issues Mol Biol. 2022. PMID: 36005147 Free PMC article.
-
Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement.Infect Immun. 2007 Aug;75(8):3791-801. doi: 10.1128/IAI.01779-06. Epub 2007 May 21. Infect Immun. 2007. PMID: 17517863 Free PMC article.
References
-
- Schlech WFI. Foodborne listeriosis. Clin Infect Dis. 2000;31:770–5. - PubMed
-
- Rebhun WC. Listeriosis. Vet Clin North Am Food Anim Pract. 1987;3:75–83. - PubMed
-
- Parham P, Unanue ER, editors. Immunol Rev. Vol. 158. 1997. Immunity to Listeria monocytogenes: a model intracellular pathogen; pp. 11–70.
-
- Unanue ER. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res Immunol. 1996;147:499–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

