Non-responsiveness to hepatitis B surface antigen vaccines is not caused by defective antigen presentation or a lack of B7 co-stimulation
- PMID: 15762884
- PMCID: PMC1809328
- DOI: 10.1111/j.1365-2249.2004.02749.x
Non-responsiveness to hepatitis B surface antigen vaccines is not caused by defective antigen presentation or a lack of B7 co-stimulation
Abstract
The mechanisms causing non-responsiveness to hepatitis B surface antigen (HBsAg) vaccines in man remain elusive. The increased incidence of non-responsiveness in subjects with HLA-DR3(+) or -DR7(+) haplotypes suggests that immune response mechanisms governed by genes of the MHC are involved. Homozygotes for these two haplotypes are found almost exclusively in the non-responder (NR) population. It is conceivable that antigen-presenting cells (APC) of NR are defective in the uptake of HBsAg and that they are unable to present this Ag adequately. Previously, we demonstrated that DR2(+), DR7(+) and DP4(+) NR were able to present HBsAg. In the present paper we demonstrate that six DR0301(+) NR, five of which are homozygous for this marker, were able to take up, process and present HBsAg to HBsAg-specific, DR0301-restricted T cell lines. Non-fractionated peripheral blood mononuclear cells (PBMC) from the DR0301(+) NR did not proliferate to HBsAg in vitro, whereas they proliferated vigorously upon stimulation with tetanus toxoid, thus ruling out the presence of a generalized immunodeficiency. We therefore conclude that HLA-DR0301(+) NR vaccinees are not deficient in their HBsAg-presentation. Because it was demonstrated that recently activated T cells can apparently bypass the requirement for B7, we may have overlooked the role of the B7-co-stimulation in our set-up that used HBsAg-specific T cell lines. Therefore we examined the expression of B7 co-stimulatory molecules on NR-APC. CD86 was normally present on these cells and was not down-regulated after culturing the PBMC in the presence of HBsAg. We conclude that CD86 expression on CD14(+) monocytes of DR0301- and DR07-homozygous poor responders is not deficient and cannot be the mechanism underlying the non-responsiveness of these subjects.
Figures
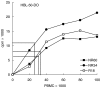
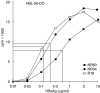


References
-
- Craven DE, Awdeh ZL, Kunches LM, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med. 1986;105:356–60. - PubMed
-
- Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196–204. - PubMed
-
- Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982;307:1481–6. - PubMed
-
- Walker M, Szmuness W, Stevens CE, Rubinstein P. Genetics of anti-HBs responsiveness. I. HLA-DR7 and nonresponsiveness to hepatitis vaccination. Transfusion. 1981;21:601.
-
- Milich DR, Leroux-Roels GG. Immunogenetics of the response to HBsAg vaccination. Autoimmun Rev. 2003;2:248–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

