Analyses of the differentiation potential of satellite cells from myoD-/-, mdx, and PMP22 C22 mice
- PMID: 15762989
- PMCID: PMC1079863
- DOI: 10.1186/1471-2474-6-15
Analyses of the differentiation potential of satellite cells from myoD-/-, mdx, and PMP22 C22 mice
Abstract
Background: Sporadic and sometimes contradictory studies have indicated changes in satellite cell behaviour associated with the progressive nature of human Duchenne muscular dystrophy (DMD). Satellite cell proliferation and number are reportedly altered in DMD and the mdx mouse model. We recently found that satellite cells in MSVski transgenic mice, a muscle hypertrophy model showing progressive muscle degeneration, display a severe ageing-related differentiation defect in vitro. We tested the hypothesis that similar changes contribute to the gradual loss of muscle function with age in mdx and PMP22 mice, a model of human motor and sensory neuropathy type 1A (HMSN1A).
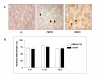
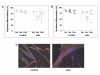
Methods: Single extensor digitorum longus muscle fibres were cultured from mdx and PMP22 mice and age- and genetic background-matched controls. Mice at several ages were compared with regard to the differentiation of satellite cells, assayed as the proportion of desmin-expressing cells that accumulated sarcomeric myosin heavy chain.
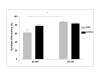
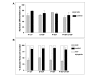
Results: Satellite cells of 2 month, 6 month, and 12 month old mdx mice were capable of differentiating to a similar extent to age-matched wild type control animals in an in vitro proliferation/differentiation model. Strikingly, differentiation efficiency in individual 6 month and 12 month old mdx animals varies to a much higher extent than in age-matched controls, younger mdx animals, or PMP22 mice. In contrast, differentiation of myoblasts from all myoD null mice assayed was severely impaired in this assay system. The defect in satellite cell differentiation that occurs in some mdx animals arises from a delay in differentiation that is not overcome by IGF-1 treatment at any phase of cultivation.
Conclusion: Overall, a defect in satellite cell differentiation above that arising through normal ageing does not occur in mdx or PMP22 mouse models of human disease. Nonetheless, the impaired differentiation of satellite cells from some mdx animals suggests that additional factors, environmental or epigenetic, may lead to deteriorating muscle repair through poor differentiation of satellite cells in genetically predisposed individuals.
Figures

Similar articles
-
miR-146a deficiency does not aggravate muscular dystrophy in mdx mice.Skelet Muscle. 2019 Aug 14;9(1):22. doi: 10.1186/s13395-019-0207-0. Skelet Muscle. 2019. PMID: 31412923 Free PMC article.
-
Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction.Am J Physiol Cell Physiol. 2007 Aug;293(2):C661-9. doi: 10.1152/ajpcell.00061.2007. Epub 2007 May 2. Am J Physiol Cell Physiol. 2007. PMID: 17475662
-
Satellite cells and utrophin are not directly correlated with the degree of skeletal muscle damage in mdx mice.Am J Physiol Cell Physiol. 2005 Jul;289(1):C42-8. doi: 10.1152/ajpcell.00577.2004. Epub 2005 Feb 9. Am J Physiol Cell Physiol. 2005. PMID: 15703201
-
Severe cardiomyopathy in mice lacking dystrophin and MyoD.Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):220-5. doi: 10.1073/pnas.96.1.220. Proc Natl Acad Sci U S A. 1999. PMID: 9874799 Free PMC article.
-
Diarylpropionitrile-stimulated ERβ nuclear accumulation promotes MyoD-induced muscle regeneration in mdx mice by interacting with FOXO3A.Pharmacol Res. 2024 Oct;208:107376. doi: 10.1016/j.phrs.2024.107376. Epub 2024 Aug 30. Pharmacol Res. 2024. PMID: 39216837
Cited by
-
Non-passaged muscle precursor cells from 32-month old rat skeletal muscle have delayed proliferation and differentiation.Cell Prolif. 2013 Feb;46(1):45-57. doi: 10.1111/cpr.12007. Epub 2012 Dec 21. Cell Prolif. 2013. PMID: 23279018 Free PMC article.
-
MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration.Genes Dev. 2012 Jan 15;26(2):190-202. doi: 10.1101/gad.179663.111. Genes Dev. 2012. PMID: 22279050 Free PMC article.
-
Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates.J Histochem Cytochem. 2011 Jan;59(1):33-46. doi: 10.1369/jhc.2010.956995. J Histochem Cytochem. 2011. PMID: 21339173 Free PMC article.
-
Loss of full-length dystrophin expression results in major cell-autonomous abnormalities in proliferating myoblasts.Elife. 2022 Sep 27;11:e75521. doi: 10.7554/eLife.75521. Elife. 2022. PMID: 36164827 Free PMC article.
-
Human myotube formation is determined by MyoD-Myomixer/Myomaker axis.Sci Adv. 2020 Dec 18;6(51):eabc4062. doi: 10.1126/sciadv.abc4062. Print 2020 Dec. Sci Adv. 2020. PMID: 33355126 Free PMC article.
References
-
- Hoffmann EP. In: Dystrophinopathies. Karpati GHJDGRC, editor. Cambridge University Press; 2001. pp. 385–432. (Disorders of Voluntary Muscle).
-
- Nigro V, Nigro G, Esposito MG, Comi LI, Molinari AM, Puca GA, Politano L. Novel small mutations along the DMD/BMD gene associated with different phenotypes. Hum Mol Genet. 1994;3:1907–1908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

