Two cellular proteins that interact with a stem loop in the simian hemorrhagic fever virus 3'(+)NCR RNA
- PMID: 15763141
- PMCID: PMC7126611
- DOI: 10.1016/j.virusres.2004.11.014
Two cellular proteins that interact with a stem loop in the simian hemorrhagic fever virus 3'(+)NCR RNA
Abstract
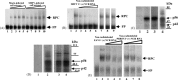
Both full-length and subgenomic negative-strand RNAs are initiated at the 3' terminus of the positive-strand genomic RNA of the arterivirus, simian hemorrhagic fever virus (SHFV). The SHFV 3'(+) non-coding region (NCR) is 76 nts in length and forms a stem loop (SL) structure that was confirmed by ribonuclease structure probing. Two cell proteins, p56 and p42, bound specifically to a probe consisting of the SHFV 3'(+)NCR RNA. The 3'(+)NCR RNAs of two additional members of the arterivirus genus specifically interacted with two cell proteins of the same size. p56 was identified as polypyrimidine tract-binding protein (PTB) and p42 was identified as fructose bisphosphate aldolase A. PTB binding sites were mapped to a terminal loop and to a bulged region of the SHFV 3'SL structure. Deletion of either of the PTB binding sites in the viral RNA significantly reduced PTB binding activity, suggesting that both sites are required for efficient binding of this protein. Changes in the top portion of the SHFV 3'SL structure eliminated aldolase binding, suggesting that the binding site for this protein is located near the top of the SL. These cell proteins may play roles in regulating the functions of the genomic 3' NCR.
Figures




Similar articles
-
Simian hemorrhagic fever virus: Recent advances.Virus Res. 2015 Apr 16;202:112-9. doi: 10.1016/j.virusres.2014.11.024. Epub 2014 Nov 29. Virus Res. 2015. PMID: 25455336 Free PMC article. Review.
-
A 68-nucleotide sequence within the 3' noncoding region of simian hemorrhagic fever virus negative-strand RNA binds to four MA104 cell proteins.J Virol. 1998 May;72(5):4341-51. doi: 10.1128/JVI.72.5.4341-4351.1998. J Virol. 1998. PMID: 9557724 Free PMC article.
-
Cell proteins bind to a 67 nucleotide sequence within the 3' noncoding region (NCR) of simian hemorrhagic fever virus (SHFV) negative-strand RNA.Adv Exp Med Biol. 1998;440:235-40. doi: 10.1007/978-1-4615-5331-1_29. Adv Exp Med Biol. 1998. PMID: 9782286
-
Identification of cell proteins that bind to the SHFV 3' (+)NCR.Adv Exp Med Biol. 2001;494:647-53. doi: 10.1007/978-1-4615-1325-4_96. Adv Exp Med Biol. 2001. PMID: 11774540 No abstract available.
-
Towards an Ideal In Cell Hybridization-Based Strategy to Discover Protein Interactomes of Selected RNA Molecules.Int J Mol Sci. 2022 Jan 15;23(2):942. doi: 10.3390/ijms23020942. Int J Mol Sci. 2022. PMID: 35055128 Free PMC article. Review.
Cited by
-
Simian hemorrhagic fever virus: Recent advances.Virus Res. 2015 Apr 16;202:112-9. doi: 10.1016/j.virusres.2014.11.024. Epub 2014 Nov 29. Virus Res. 2015. PMID: 25455336 Free PMC article. Review.
-
Cis-acting RNA elements in human and animal plus-strand RNA viruses.Biochim Biophys Acta. 2009 Sep-Oct;1789(9-10):495-517. doi: 10.1016/j.bbagrm.2009.09.007. Epub 2009 Sep 23. Biochim Biophys Acta. 2009. PMID: 19781674 Free PMC article. Review.
-
Genetic diversity in the yellow head nidovirus complex.Virology. 2008 Oct 25;380(2):213-25. doi: 10.1016/j.virol.2008.07.005. Epub 2008 Sep 2. Virology. 2008. PMID: 18768192 Free PMC article.
-
An interactome map of the nucleocapsid protein from a highly pathogenic North American porcine reproductive and respiratory syndrome virus strain generated using SILAC-based quantitative proteomics.Proteomics. 2012 Apr;12(7):1015-23. doi: 10.1002/pmic.201100469. Proteomics. 2012. PMID: 22522808 Free PMC article.
-
Structures and Functions of the 3' Untranslated Regions of Positive-Sense Single-Stranded RNA Viruses Infecting Humans and Animals.Front Cell Infect Microbiol. 2020 Aug 27;10:453. doi: 10.3389/fcimb.2020.00453. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974223 Free PMC article. Review.
References
-
- Blyn L.B., Swiderek K.M., Richards O., Stahl D.C., Semler B.L., Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ non-coding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11115–11120. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

