Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map
- PMID: 15764651
- PMCID: PMC1305609
- DOI: 10.1529/biophysj.104.058727
Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map
Abstract
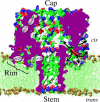
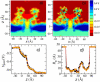
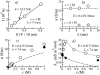
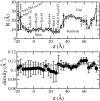
alpha-Hemolysin of Staphylococcus aureus is a self-assembling toxin that forms a water-filled transmembrane channel upon oligomerization in a lipid membrane. Apart from being one of the best-studied toxins of bacterial origin, alpha-hemolysin is the principal component in several biotechnological applications, including systems for controlled delivery of small solutes across lipid membranes, stochastic sensors for small solutes, and an alternative to conventional technology for DNA sequencing. Through large-scale molecular dynamics simulations, we studied the permeability of the alpha-hemolysin/lipid bilayer complex for water and ions. The studied system, composed of approximately 300,000 atoms, included one copy of the protein, a patch of a DPPC lipid bilayer, and a 1 M water solution of KCl. Monitoring the fluctuations of the pore structure revealed an asymmetric, on average, cross section of the alpha-hemolysin stem. Applying external electrostatic fields produced a transmembrane ionic current; repeating simulations at several voltage biases yielded a current/voltage curve of alpha-hemolysin and a set of electrostatic potential maps. The selectivity of alpha-hemolysin to Cl(-) was found to depend on the direction and the magnitude of the applied voltage bias. The results of our simulations are in excellent quantitative agreement with available experimental data. Analyzing trajectories of all water molecule, we computed the alpha-hemolysin's osmotic permeability for water as well as its electroosmotic effect, and characterized the permeability of its seven side channels. The side channels were found to connect seven His-144 residues surrounding the stem of the protein to the bulk solution; the protonation of these residues was observed to affect the ion conductance, suggesting the seven His-144 to comprise the pH sensor that gates conductance of the alpha-hemolysin channel.
Figures
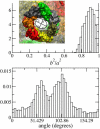
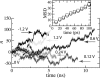
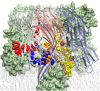
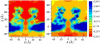
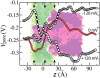
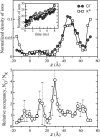
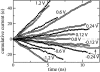
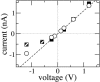

Comment in
-
Explicit channel conductance: can it be computed?Biophys J. 2005 Jun;88(6):3742-3. doi: 10.1529/biophysj.105.060996. Epub 2005 Mar 11. Biophys J. 2005. PMID: 15764668 Free PMC article. No abstract available.
References
-
- Allen, M. P., and D. J. Tildesley. 1987. Computer Simulation of Liquids. Oxford University Press, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

