STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFbeta-activated kinase 1 for STAT3 Ser-727 phosphorylation
- PMID: 15764709
- PMCID: PMC555521
- DOI: 10.1073/pnas.0500679102
STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFbeta-activated kinase 1 for STAT3 Ser-727 phosphorylation
Abstract
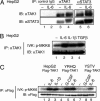
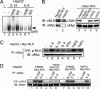
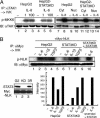
Signal transducer and activator of transcription 3 (STAT3) is activated by the IL-6 family of cytokines and growth factors. STAT3 requires phosphorylation on Ser-727, in addition to tyrosine phosphorylation on Tyr-705, to be transcriptionally active. In IL-6 signaling, the two major pathways that derive from the YXXQ and the YSTV motifs of gp130 cause Ser-727 phosphorylation. Here, we show that TGF-beta-activated kinase 1 (TAK1) interacts with STAT3, that the TAK1-Nemo-like kinase (NLK) pathway is efficiently activated by IL-6 through the YXXQ motif, and that this is the YXXQ-mediated H7-sensitive pathway that leads to STAT3 Ser-727 phosphorylation. Because NLK was recently shown to interact with STAT3, we explored the role of STAT3 in activating this pathway. Depletion of STAT3 diminished the IL-6-induced NLK activation by >80% without inhibiting IL-6-induced TAK1 activation or its nuclear entry. We found that STAT3 functioned as a scaffold for TAK1 and NLK in vivo through a region in its carboxyl terminus. Furthermore, the expression of the STAT3(534-770) region in the nuclei of STAT3-knockdown cells enhanced the IL-6-induced NLK activation in a dose-dependent manner but not the TGFbeta-induced NLK activation. TGFbeta did not cause STAT3 Ser-727 phosphorylation, even when the carboxyl region of STAT3 was expressed in the nuclei. Together, these results indicate that STAT3 enhances the efficiency of its own Ser-727 phosphorylation by acting as a scaffold for the TAK1-NLK kinases, specifically in the YXXQ motif-derived pathway.
Figures


References
-
- Levy, D. E. & Darnell, J. E., Jr. (2002) Nat. Rev. Mol. Cell. Biol. 3, 651–662. - PubMed
-
- Decker, T. & Kovarik, P. (2000) Oncogene 19, 2628–2637. - PubMed
-
- Wen, Z., Zhong, Z. & Darnell, J. E., Jr. (1995) Cell 82, 241–250. - PubMed
-
- Abe, K., Hirai, M., Mizuno, K., Higashi, N., Sekimoto, T., Miki, T., Hirano, T. & Nakajima, K. (2001) Oncogene 20, 3464–3474. - PubMed
-
- Pesu, M., Takaluoma, K., Aittomaki, S., Lagerstedt, A., Saksela, K., Kovanen, P. E. & Silvennoinen, O. (2000) Blood 95, 494–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

