NO/redox disequilibrium in the failing heart and cardiovascular system
- PMID: 15765132
- PMCID: PMC1052013
- DOI: 10.1172/JCI24459
NO/redox disequilibrium in the failing heart and cardiovascular system
Abstract
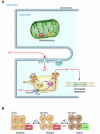
There is growing evidence that the altered production and/or spatiotemporal distribution of reactive oxygen and nitrogen species creates oxidative and/or nitrosative stresses in the failing heart and vascular tree, which contribute to the abnormal cardiac and vascular phenotypes that characterize the failing cardiovascular system. These derangements at the integrated system level can be interpreted at the cellular and molecular levels in terms of adverse effects on signaling elements in the heart, vasculature, and blood that subserve cardiac and vascular homeostasis.
Figures


Similar articles
-
Nitric oxide in blood. The nitrosative-oxidative disequilibrium hypothesis on the pathogenesis of cardiovascular disease.FEBS J. 2007 Feb;274(4):906-23. doi: 10.1111/j.1742-4658.2007.05660.x. Epub 2007 Jan 22. FEBS J. 2007. PMID: 17244198 Review.
-
Nitric oxide and cell signaling: modulation of redox tone and protein modification.Amino Acids. 2003 Dec;25(3-4):313-21. doi: 10.1007/s00726-003-0019-7. Epub 2003 Aug 28. Amino Acids. 2003. PMID: 14661093 Review.
-
Redox-mediated signal transduction by cardiovascular Nox NADPH oxidases.J Mol Cell Cardiol. 2014 Aug;73:70-9. doi: 10.1016/j.yjmcc.2014.02.006. Epub 2014 Feb 19. J Mol Cell Cardiol. 2014. PMID: 24560815 Review.
-
Redox regulation of cardiovascular inflammation - Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species.Free Radic Biol Med. 2017 Aug;109:48-60. doi: 10.1016/j.freeradbiomed.2017.01.027. Epub 2017 Jan 18. Free Radic Biol Med. 2017. PMID: 28108279 Review.
-
Oxygen, oxidative stress, hypoxia, and heart failure.J Clin Invest. 2005 Mar;115(3):500-8. doi: 10.1172/JCI24408. J Clin Invest. 2005. PMID: 15765131 Free PMC article. Review.
Cited by
-
Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery.Chem Rev. 2013 Jul 10;113(7):4633-79. doi: 10.1021/cr300163e. Epub 2013 Mar 20. Chem Rev. 2013. PMID: 23514336 Free PMC article. Review. No abstract available.
-
Implications of Oxidative and Nitrosative Post-Translational Modifications in Therapeutic Strategies against Reperfusion Damage.Antioxidants (Basel). 2021 May 8;10(5):749. doi: 10.3390/antiox10050749. Antioxidants (Basel). 2021. PMID: 34066806 Free PMC article. Review.
-
Alterations of tumor microenvironment by nitric oxide impedes castration-resistant prostate cancer growth.Proc Natl Acad Sci U S A. 2018 Oct 30;115(44):11298-11303. doi: 10.1073/pnas.1812704115. Epub 2018 Oct 15. Proc Natl Acad Sci U S A. 2018. PMID: 30322928 Free PMC article.
-
Deletion of metallothionein exacerbates intermittent hypoxia-induced oxidative and inflammatory injury in aorta.Oxid Med Cell Longev. 2014;2014:141053. doi: 10.1155/2014/141053. Epub 2014 Aug 6. Oxid Med Cell Longev. 2014. PMID: 25177426 Free PMC article.
-
Molecular Mechanisms Underlying Heart Failure and Their Therapeutic Potential.Cells. 2025 Feb 20;14(5):324. doi: 10.3390/cells14050324. Cells. 2025. PMID: 40072053 Free PMC article. Review.
References
-
- McCord JM, Fridovitch I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753–5760. - PubMed
-
- McCord JM, Fridovitch I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. - PubMed
-
- Keith M, et al. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:1352–1356. - PubMed
-
- Cesselli D, et al. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001;89:279–286. - PubMed
-
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996;28:506–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

