Toward transcriptional therapies for the failing heart: chemical screens to modulate genes
- PMID: 15765135
- PMCID: PMC1052006
- DOI: 10.1172/JCI24144
Toward transcriptional therapies for the failing heart: chemical screens to modulate genes
Abstract
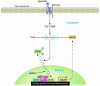
In response to acute and chronic stresses, the heart frequently undergoes a remodeling process that is accompanied by myocyte hypertrophy, impaired contractility, and pump failure, often culminating in sudden death. The existence of redundant signaling pathways that trigger heart failure poses challenges for therapeutic intervention. Cardiac remodeling is associated with the activation of a pathological gene program that weakens cardiac performance. Thus, targeting the disease process at the level of gene expression represents a potentially powerful therapeutic approach. In this review, we describe strategies for normalizing gene expression in the failing heart with small molecules that control signal transduction pathways directed at transcription factors and associated chromatin-modifying enzymes.
Figures







Similar articles
-
Control of cardiac hypertrophy and heart failure by histone acetylation/deacetylation.Novartis Found Symp. 2006;274:3-12; discussion 13-9, 152-5, 272-6. Novartis Found Symp. 2006. PMID: 17019803 Review.
-
Control of cardiac growth by histone acetylation/deacetylation.Circ Res. 2006 Jan 6;98(1):15-24. doi: 10.1161/01.RES.0000197782.21444.8f. Circ Res. 2006. PMID: 16397154 Review.
-
Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes.Cardiovasc Res. 2004 Aug 15;63(3):450-7. doi: 10.1016/j.cardiores.2004.04.002. Cardiovasc Res. 2004. PMID: 15276470 Review.
-
Targeting histone deacetylases for heart failure.Expert Opin Ther Targets. 2009 Jul;13(7):767-84. doi: 10.1517/14728220902939161. Expert Opin Ther Targets. 2009. PMID: 19466913 Review.
-
Derepression of pathological cardiac genes by members of the CaM kinase superfamily.Cardiovasc Res. 2007 Mar 1;73(4):667-77. doi: 10.1016/j.cardiores.2006.11.036. Epub 2006 Dec 5. Cardiovasc Res. 2007. PMID: 17217938 Review.
Cited by
-
A pathway involving HDAC5, cFLIP and caspases regulates expression of the splicing regulator polypyrimidine tract binding protein in the heart.J Cell Sci. 2013 Apr 1;126(Pt 7):1682-91. doi: 10.1242/jcs.121384. Epub 2013 Feb 19. J Cell Sci. 2013. PMID: 23424201 Free PMC article.
-
Inhibition of class I histone deacetylase activity represses matrix metalloproteinase-2 and -9 expression and preserves LV function postmyocardial infarction.Am J Physiol Heart Circ Physiol. 2015 Jun 1;308(11):H1391-401. doi: 10.1152/ajpheart.00390.2014. Epub 2015 Mar 20. Am J Physiol Heart Circ Physiol. 2015. PMID: 25795711 Free PMC article.
-
The cardioprotective effect of dexrazoxane (Cardioxane) is consistent with sequestration of poly(ADP-ribose) by self-assembly and not depletion of topoisomerase 2B.Ecancermedicalscience. 2018 Dec 20;12:889. doi: 10.3332/ecancer.2018.889. eCollection 2018. Ecancermedicalscience. 2018. PMID: 30792806 Free PMC article.
-
Short-term effects of pressure overload on the expression of genes involved in calcium homeostasis.Mol Cell Biochem. 2008 Jun;313(1-2):29-36. doi: 10.1007/s11010-008-9738-0. Epub 2008 Mar 26. Mol Cell Biochem. 2008. PMID: 18365304
-
The role of nuclear factor of activated T cells in pulmonary arterial hypertension.Cell Cycle. 2017 Mar 19;16(6):508-514. doi: 10.1080/15384101.2017.1281485. Epub 2017 Jan 19. Cell Cycle. 2017. PMID: 28103134 Free PMC article. Review.
References
-
- Linseman JV, Bristow MR. Drug therapy and heart failure prevention. Circulation. 2003;107:1234–1236. - PubMed
-
- Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am. Heart J. 2001;141:334–341. - PubMed
-
- Okin PM, et al. for the LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. - PubMed
-
- Devereux RB, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. - PubMed
-
- Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004;292:2396–2398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

