Altered intracellular Ca2+ handling in heart failure
- PMID: 15765137
- PMCID: PMC1052007
- DOI: 10.1172/JCI24159
Altered intracellular Ca2+ handling in heart failure
Abstract
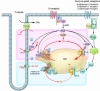
Structural and functional alterations in the Ca2+ regulatory proteins present in the sarcoplasmic reticulum have recently been shown to be strongly involved in the pathogenesis of heart failure. Chronic activation of the sympathetic nervous system or of the renin-angiotensin system induces abnormalities in both the function and structure of these proteins. We review here the considerable body of evidence that has accumulated to support the notion that such abnormalities contribute to a defectiveness of contractile performance and hence to the progression of heart failure.
Figures

References
-
- Francis GS. Pathophysiology of chronic heart failure. Am. J. Med. 2001;110:37S–46S. - PubMed
-
- Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–IV23. - PubMed
-
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002;34:951–969. - PubMed
-
- Meyer M, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. - PubMed
-
- Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ. Res. 2003;92:350–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

