Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors
- PMID: 15765148
- PMCID: PMC1051987
- DOI: 10.1172/JCI22655
Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors
Abstract
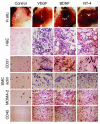
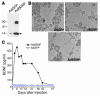
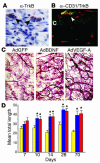
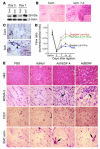
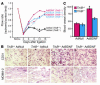
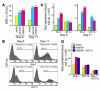
The neurotrophin brain-derived neurotrophic factor (BDNF) is required for the maintenance of cardiac vessel wall stability during embryonic development through direct angiogenic actions on endothelial cells expressing the tropomysin receptor kinase B (TrkB). However, the role of BDNF and a related neurotrophin ligand, neurotrophin-4 (NT-4), in the regulation of revascularization of the adult tissues is unknown. To study the potential angiogenic capacity of BDNF in mediating the neovascularization of ischemic and non-ischemic adult mouse tissues, we utilized a hindlimb ischemia and a subcutaneous Matrigel model. Recruitment of endothelial cells and promotion of channel formation within the Matrigel plug by BDNF and NT-4 was comparable to that induced by VEGF-A. The introduction of BDNF into non-ischemic ears or ischemic limbs induced neoangiogenesis, with a 2-fold increase in the capillary density. Remarkably, treatment with BDNF progressively increased blood flow in the ischemic limb over 21 days, similar to treatment with VEGF-A. The mechanism by which BDNF enhances capillary formation is mediated in part through local activation of the TrkB receptor and also by recruitment of Sca-1+CD11b+ pro-angiogenic hematopoietic cells. BDNF induces a potent direct chemokinetic action on subsets of marrow-derived Sca-1+ hematopoietic cells co-expressing TrkB. These studies suggest that local regional delivery of BDNF may provide a novel mechanism for inducing neoangiogenesis through both direct actions on local TrkB-expressing endothelial cells in skeletal muscle and recruitment of specific subsets of TrkB+ bone marrow-derived hematopoietic cells to provide peri-endothelial support for the newly formed vessels.
Figures

Comment in
-
Pleiotropy of tissue-specific growth factors: from neurons to vessels via the bone marrow.J Clin Invest. 2005 Mar;115(3):596-8. doi: 10.1172/JCI24511. J Clin Invest. 2005. PMID: 15765145 Free PMC article.
References
-
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. - PubMed
-
- Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. - PubMed
-
- Cao R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003;9:604–613. - PubMed
-
- Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003;9:1370–1376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

