Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice
- PMID: 15765150
- PMCID: PMC1052003
- DOI: 10.1172/JCI23625
Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice
Abstract
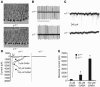
Essential tremor is the most common movement disorder and has an unknown etiology. Here we report that gamma-aminobutyric acidA (GABA(A)) receptor alpha1-/- mice exhibit postural and kinetic tremor and motor incoordination that is characteristic of essential tremor disease. We tested mice with essential-like tremor using current drug therapies that alleviate symptoms in essential tremor patients (primidone, propranolol, and gabapentin) and several candidates hypothesized to reduce tremor, including ethanol; the noncompetitive N-methyl-D-aspartate receptor antagonist MK-801; the adenosine A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA); the GABA(A) receptor modulators diazepam, allopregnanolone, and Ro15-4513; and the L-type Ca2+ channel antagonist nitrendipine. Primidone, propranolol, and gabapentin reduced the amplitude (power) of the pathologic tremor. Nonsedative doses of ethanol eliminated tremor in mice. Diazepam, allopregnanolone, Ro15-4513, and nitrendipine had no effect or enhanced tremor, whereas MK-801 and CCPA reduced tremor. To understand the etiology of tremor in these mice, we studied the electrophysiological properties of cerebellar Purkinje cells. Cerebellar Purkinje cells in GABA(A) receptor alpha1-/- mice exhibited a profound loss of all responses to synaptic or exogenous GABA, but no differences in abundance, gross morphology, or spontaneous synaptic activity were observed. This genetic animal model elucidates a mechanism of GABAergic dysfunction in the major motor pathway and potential targets for pharmacotherapy of essential tremor.
Figures

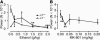
Comment in
-
Genetic mouse models of essential tremor: are they essential?J Clin Invest. 2005 Mar;115(3):584-6. doi: 10.1172/JCI24544. J Clin Invest. 2005. PMID: 15765140 Free PMC article.
References
-
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov. Disord. 1998;13:5–10. - PubMed
-
- Chen JJ, Swope DM. Essential tremor: diagnosis and treatment. Pharmacotherapy. 2003;23:1105–1122. - PubMed
-
- Pahwa R, Lyons KE. Essential tremor: Differential diagnosis and current therapy. Am. J. Med. 2003;115:134–142. - PubMed
-
- Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age. Mov. Disord. 1997;12:969–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

