Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen
- PMID: 15766662
- PMCID: PMC2846360
- DOI: 10.1016/j.ccr.2005.01.027
Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen
Abstract
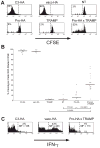
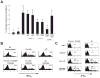
To understand the T cell response to prostate cancer, we created transgenic mice that express a model antigen in a prostate-restricted pattern and crossed these animals to TRAMP mice that develop spontaneous prostate cancer. Adoptive transfer of prostate-specific CD4 T cells shows that, in the absence of prostate cancer, the prostate gland is mostly ignored. Tumorigenesis allows T cell recognition of the prostate gland--but this recognition is tolerogenic, resulting in abortive proliferation and ultimately in hyporesponsiveness at the systemic level. Androgen ablation (the most common treatment for metastatic prostate cancer) was able to mitigate this tolerance--allowing prostate-specific T cells to expand and develop effector function after vaccination. These results suggest that immunotherapy for prostate cancer may be most efficacious when administered after androgen ablation.
Figures



References
-
- Adler AJ, Scheller A, Hoffman Y, Robins DM. Multiple components of a complex androgen-dependent enhancer. Mol Endocrinol. 1991;5:1587–1596. - PubMed
-
- Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

