Dynamics of leaf and root growth: endogenous control versus environmental impact
- PMID: 15767269
- PMCID: PMC4246750
- DOI: 10.1093/aob/mci103
Dynamics of leaf and root growth: endogenous control versus environmental impact
Abstract
Aims: Production of biomass and yield in natural and agronomic conditions depend on the endogenous growth capacity of plants and on the environmental conditions constraining it. Sink growth drives the competition for carbon, nutrients and water within the plant, and determines the structure of leaves and roots that supply resources to the plant later on. For their outstanding importance, analyses of internal growth mechanisms and of environmental impact on plant growth are long-standing topics in plant sciences.
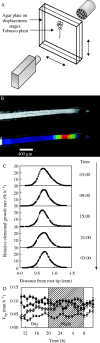
Scope: Recent technological developments have made it feasible to study the dynamics of plant growth in temporal and spatial scales that are relevant to link macroscopic growth with molecular control. These developments provided first insights into the truly dynamic interaction between environment and endogenous control of plant growth.
Conclusions: Evidence is presented in this paper that the relative importance of endogenous control versus the impact of the dynamics of the environment depends on the frequency pattern of the environmental conditions to which the tissue is exposed. It can further be speculated that this is not only relevant within individual plants (hence leaves versus roots), but also crucial for the adaptation of plant species to the various dynamics of their environments. The following are discussed: mechanisms linking growth and concentrations of primary metabolites, and differences and homologies between spatial and temporal patterns of root and leaf growth with metabolite patterns.
Figures



Similar articles
-
Environmental effects on spatial and temporal patterns of leaf and root growth.Annu Rev Plant Biol. 2009;60:279-304. doi: 10.1146/annurev.arplant.59.032607.092819. Annu Rev Plant Biol. 2009. PMID: 19575584 Review.
-
Diel patterns of leaf and root growth: endogenous rhythmicity or environmental response?J Exp Bot. 2012 May;63(9):3339-51. doi: 10.1093/jxb/err334. Epub 2012 Jan 5. J Exp Bot. 2012. PMID: 22223810 Review.
-
Environmental variation drives the decoupling of leaf and root traits within species along an elevation gradient.Ann Bot. 2022 Sep 19;130(3):419-430. doi: 10.1093/aob/mcac052. Ann Bot. 2022. PMID: 35405006 Free PMC article.
-
Asymmetric pruning reveals how organ connectivity alters the functional balance between leaves and roots of Chinese fir.J Exp Bot. 2019 Mar 27;70(6):1941-1953. doi: 10.1093/jxb/erz013. J Exp Bot. 2019. PMID: 30689933
-
Root cooling strongly affects diel leaf growth dynamics, water and carbohydrate relations in Ricinus communis.Plant Cell Environ. 2010 Mar;33(3):408-17. doi: 10.1111/j.1365-3040.2009.02090.x. Epub 2009 Nov 24. Plant Cell Environ. 2010. PMID: 19968824
Cited by
-
Glycine max leaflets lack a base-tip gradient in growth rate.J Plant Res. 2005 Oct;118(5):343-6. doi: 10.1007/s10265-005-0227-1. Epub 2005 Sep 1. J Plant Res. 2005. PMID: 16136362
-
Synchronous high-resolution phenotyping of leaf and root growth in Nicotiana tabacum over 24-h periods with GROWMAP-plant.Plant Methods. 2013 Jan 23;9(1):2. doi: 10.1186/1746-4811-9-2. Plant Methods. 2013. PMID: 23343327 Free PMC article.
-
Nocturnal changes in leaf growth of Populus deltoides are controlled by cytoplasmic growth.Planta. 2006 May;223(6):1315-28. doi: 10.1007/s00425-005-0181-0. Epub 2005 Dec 7. Planta. 2006. PMID: 16333638
-
All roads lead to growth: imaging-based and biochemical methods to measure plant growth.J Exp Bot. 2020 Jan 1;71(1):11-21. doi: 10.1093/jxb/erz406. J Exp Bot. 2020. PMID: 31613967 Free PMC article.
-
Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage.BMC Plant Biol. 2019 May 23;19(1):216. doi: 10.1186/s12870-019-1828-5. BMC Plant Biol. 2019. PMID: 31122195 Free PMC article.
References
-
- Avery GS. 1933. Structure and development of tobacco leaves. American Journal of Botany 20: 565–592.
-
- Beemster GTS, Fiorani F, Inze D. 2003. Cell cycle: the key to plant growth control? Trends in Plant Science 8: 154–158. - PubMed
-
- Bigün J, Granlund GH. 1987. Optimal orientation detection of linear symmetry. In: Proceedings of the First International Conference on Computer Vision. London, UK, 8–11 June 1987.

