CFTR gating I: Characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (DeltaR-CFTR)
- PMID: 15767295
- PMCID: PMC1382195
- DOI: 10.1085/jgp.200409227
CFTR gating I: Characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (DeltaR-CFTR)
Abstract
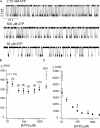
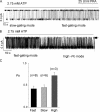
The CFTR chloride channel is activated by phosphorylation of serine residues in the regulatory (R) domain and then gated by ATP binding and hydrolysis at the nucleotide binding domains (NBDs). Studies of the ATP-dependent gating process in excised inside-out patches are very often hampered by channel rundown partly caused by membrane-associated phosphatases. Since the severed DeltaR-CFTR, whose R domain is completely removed, can bypass the phosphorylation-dependent regulation, this mutant channel might be a useful tool to explore the gating mechanisms of CFTR. To this end, we investigated the regulation and gating of the DeltaR-CFTR expressed in Chinese hamster ovary cells. In the cell-attached mode, basal DeltaR-CFTR currents were always obtained in the absence of cAMP agonists. Application of cAMP agonists or PMA, a PKC activator, failed to affect the activity, indicating that the activity of DeltaR-CFTR channels is indeed phosphorylation independent. Consistent with this conclusion, in excised inside-out patches, application of the catalytic subunit of PKA did not affect ATP-induced currents. Similarities of ATP-dependent gating between wild type and DeltaR-CFTR make this phosphorylation-independent mutant a useful system to explore more extensively the gating mechanisms of CFTR. Using the DeltaR-CFTR construct, we studied the inhibitory effect of ADP on CFTR gating. The Ki for ADP increases as the [ATP] is increased, suggesting a competitive mechanism of inhibition. Single channel kinetic analysis reveals a new closed state in the presence of ADP, consistent with a kinetic mechanism by which ADP binds at the same site as ATP for channel opening. Moreover, we found that the open time of the channel is shortened by as much as 54% in the presence of ADP. This unexpected result suggests another ADP binding site that modulates channel closing.
Figures








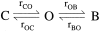
References
-
- Ai, T., S.G. Bompadre, X. Wang, S. Hu, M. Li, and T.-C. Hwang. 2004. Capsaicin potentiates wild-type and mutant CFTR chloride channel currents. Mol. Pharmacol. 65:1415–1426. - PubMed
-
- Aleksandrov, L., A.A. Aleksandrov, X.B. Chang, and J.R. Riordan. 2002. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 277:15419–15425. - PubMed
-
- Al-Nakkash, L., and T.-C. Hwang. 1999. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors. Pflugers Arch. 437:553–561. - PubMed
-
- Anderson, M.P., and M.J. Welsh. 1992. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 257:1701–1704. - PubMed

