Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1
- PMID: 15767369
- PMCID: PMC2213108
- DOI: 10.1084/jem.20042239
Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1
Abstract
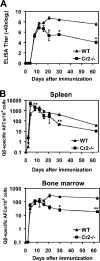
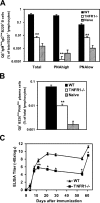
Humoral immune responses are thought to be enhanced by complement-mediated recruitment of the CD21-CD19-CD81 coreceptor complex into the B cell antigen receptor (BCR) complex, which lowers the threshold of B cell activation and increases the survival and proliferative capacity of responding B cells. To investigate the role of the CD21-CD35 complement receptors in the generation of B cell memory, we analyzed the response against viral particles derived from the bacteriophage Qbeta in mice deficient in CD21-CD35 (Cr2(-/-)). Despite highly efficient induction of early antibody responses and germinal center (GC) reactions to immunization with Qbeta, Cr2(-/-) mice exhibited impaired antibody persistence paralleled by a strongly reduced development of bone marrow plasma cells. Surprisingly, antigen-specific memory B cells were essentially normal in these mice. In the absence of CD21-mediated costimulation, Qbeta-specific post-GC B cells failed to induce the transcriptional regulators Blimp-1 and XBP-1 driving plasma cell differentiation, and the antiapoptotic protein Bcl-2, which resulted in failure to generate the precursor population of long-lived plasma cells residing in the bone marrow. These results suggest that complement receptors maintain antibody responses by delivery of differentiation and survival signals to precursors of bone marrow plasma cells.
Figures




Similar articles
-
Role of complement-binding CD21/CD19/CD81 in enhancing human B cell protection from Fas-mediated apoptosis.J Immunol. 2003 Nov 15;171(10):5244-54. doi: 10.4049/jimmunol.171.10.5244. J Immunol. 2003. PMID: 14607925
-
Optimal long-term humoral responses to replication-defective herpes simplex virus require CD21/CD35 complement receptor expression on stromal cells.J Virol. 2006 Jul;80(14):7111-7. doi: 10.1128/JVI.01421-05. J Virol. 2006. PMID: 16809316 Free PMC article.
-
Regulatory mechanisms that determine the development and function of plasma cells.Annu Rev Immunol. 2003;21:205-30. doi: 10.1146/annurev.immunol.21.120601.141138. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524387 Review.
-
Humoral immune responses in Cr2-/- mice: enhanced affinity maturation but impaired antibody persistence.J Immunol. 2000 May 1;164(9):4522-32. doi: 10.4049/jimmunol.164.9.4522. J Immunol. 2000. PMID: 10779753
-
Regulation of humoral immune responses by CD21/CD35.Immunol Rev. 2000 Aug;176:194-204. doi: 10.1034/j.1600-065x.2000.00603.x. Immunol Rev. 2000. PMID: 11043778 Free PMC article. Review.
Cited by
-
Development of a Vaccine against SARS-CoV-2 Based on the Receptor-Binding Domain Displayed on Virus-Like Particles.Vaccines (Basel). 2021 Apr 16;9(4):395. doi: 10.3390/vaccines9040395. Vaccines (Basel). 2021. PMID: 33923573 Free PMC article.
-
Somatic stem cells and the origin of cancer.Clin Transl Oncol. 2006 Sep;8(9):647-63. doi: 10.1007/s12094-006-0035-7. Clin Transl Oncol. 2006. PMID: 17005467 Review.
-
Antigen-induced B cell apoptosis is independent of complement C4.Clin Exp Immunol. 2007 Oct;150(1):132-9. doi: 10.1111/j.1365-2249.2007.03456.x. Epub 2007 Jul 23. Clin Exp Immunol. 2007. PMID: 17645767 Free PMC article.
-
Investigating the Mechanism of Germinal Center Shutdown.Front Immunol. 2022 Jul 14;13:922318. doi: 10.3389/fimmu.2022.922318. eCollection 2022. Front Immunol. 2022. PMID: 35911680 Free PMC article.
-
Immune system development varies according to age, location, and anemia in African children.Sci Transl Med. 2020 Feb 5;12(529):eaaw9522. doi: 10.1126/scitranslmed.aaw9522. Sci Transl Med. 2020. PMID: 32024802 Free PMC article.
References
-
- Nossal, G.J. 1999. Vaccines. Fundamental Immunology. W.E. Paul, editor. Lippincott-Raven, Philadelphia. 1387–1425.
-
- Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. - PubMed
-
- Bachmann, M.F., T.M. Kundig, C.P. Kalberer, H. Hengartner, and R.M. Zinkernagel. 1994. How many specific B cells are needed to protect against a virus? J. Immunol. 152:4235–4241. - PubMed
-
- MacLennan, I.C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

