Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies
- PMID: 15767393
- PMCID: PMC1061572
- DOI: 10.1128/JVI.79.7.3903-3919.2005
Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies
Abstract
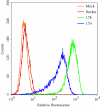
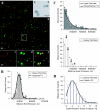
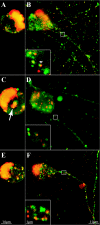
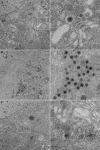
The molecular mechanisms responsible for long-distance, directional spread of alphaherpesvirus infections via axons of infected neurons are poorly understood. We describe the use of red and green fluorescent protein (GFP) fusions to capsid and tegument components, respectively, to visualize purified, single extracellular virions and axonal assemblies after pseudorabies virus (PRV) infection of cultured neurons. We observed heterogeneity in GFP fluorescence when GFP was fused to the tegument component VP22 in both single extracellular virions and discrete puncta in infected axons. This heterogeneity was observed in the presence or absence of a capsid structure detected by a fusion of monomeric red fluorescent protein to VP26. The similarity of the heterogeneous distribution of these fluorescent protein fusions in both purified virions and in axons suggested that tegument-capsid assembly and axonal targeting of viral components are linked. One possibility was that the assembly of extracellular and axonal particles containing the dually fluorescent fusion proteins occurred by the same process in the cell body. We tested this hypothesis by treating infected cultured neurons with brefeldin A, a potent inhibitor of herpesvirus maturation and secretion. Brefeldin A treatment disrupted the neuronal secretory pathway, affected fluorescent capsid and tegument transport in the cell body, and blocked subsequent entry into axons of capsid and tegument proteins. Electron microscopy demonstrated that in the absence of brefeldin A treatment, enveloped capsids entered axons, but in the presence of the inhibitor, unenveloped capsids accumulated in the cell body. These results support an assembly process in which PRV capsids acquire a membrane in the cell body prior to axonal entry and subsequent transport.
Figures





References
-
- Baron, M. D., and H. Garoff. 1990. Mannosidase II and the 135-kDa Golgi-specific antigen recognized monoclonal antibody 53FC3 are the same dimeric protein. J. Biol. Chem. 265:19928-19931. - PubMed
-
- Bestvater, F., T. A. Knoch, J. Langowski, and E. Spiess. 2002. Construct conversions caused by simultaneous co-transfection: “GFP-walking.” BioTechniques 32:844-850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

