ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export
- PMID: 15767397
- PMCID: PMC1061567
- DOI: 10.1128/JVI.79.7.3949-3961.2005
ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export
Abstract
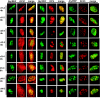
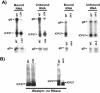
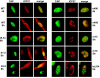
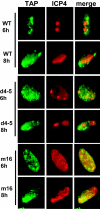
Herpes simplex virus type 1 (HSV-1) protein ICP27 interacts with the cellular export adaptor protein Aly/REF, which is part of the exon junction complex implicated in cellular mRNA export. We previously reported that Aly/REF was no longer associated with splicing factor SC35 sites during infection but instead colocalized with ICP27 in distinct structures. Here we show that these structures colocalize with ICP4 and are sites of HSV-1 transcription. ICP27 mutants with lesions in the region required for the interaction with Aly/REF failed to recruit Aly/REF to viral transcription sites; however, ICP27 export to the cytoplasm was unimpaired, indicating that the interaction of ICP27 with Aly/REF is not required for ICP27 shuttling. ICP27 has also been shown to interact with the cellular mRNA export receptor TAP/NXF1. We report that ICP27 interacts directly with TAP/NXF1 and does not require Aly/REF to bridge the interaction. The C terminus of ICP27 is required; however, the N-terminal leucine-rich region also contributes to the interaction of ICP27 with TAP/NXF1. In contrast to the results found for Aly/REF, mutants that failed to interact with TAP/NXF1 were not exported to the cytoplasm, and TAP/NXF1 was not recruited to sites of HSV-1 transcription. Therefore, the interaction of ICP27 with TAP/NXF1 occurs after ICP27 leaves viral transcription sites. We conclude that ICP27 and the viral RNAs to which it binds are exported via the TAP/NXF1 export receptor.
Figures





References
-
- Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Gorlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. - PMC - PubMed
-
- Braun, I. C., A. Herold, M. Rode, E. Conti, and E. Izaurralde. 2001. Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276:20536-20543. - PubMed
-
- Cullen, B. R. 2003. Nuclear RNA export. J. Cell Sci. 116:587-597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

