Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes
- PMID: 15767402
- PMCID: PMC1061520
- DOI: 10.1128/JVI.79.7.3998-4011.2005
Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes
Abstract
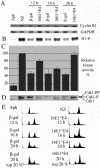
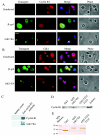
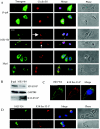
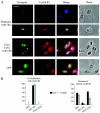
Human papillomavirus type 16 (HPV16) can cause cervical cancer. Expression of the viral E1 E4 protein is lost during malignant progression, but in premalignant lesions, E1 E4 is abundant in cells supporting viral DNA amplification. Expression of 16E1 E4 in cell culture causes G2 cell cycle arrest. Here we show that unlike many other G2 arrest mechanisms, 16E1 E4 does not inhibit the kinase activity of the Cdk1/cyclin B1 complex. Instead, 16E1 E4 uses a novel mechanism in which it sequesters Cdk1/cyclin B1 onto the cytokeratin network. This prevents the accumulation of active Cdk1/cyclin B1 complexes in the nucleus and hence prevents mitosis. A mutant 16E1 E4 (T22A, T23A) which does not bind cyclin B1 or alter its intracellular location fails to induce G2 arrest. The significance of these results is highlighted by the observation that in lesions induced by HPV16, there is evidence for Cdk1/cyclin B1 activity on the keratins of 16E1 E4-expressing cells. We hypothesize that E1 E4-induced G2 arrest may play a role in creating an environment optimal for viral DNA replication and that loss of E1 E4 expression may contribute to malignant progression.
Figures




References
-
- Bryan, J. T., and D. R. Brown. 2000. Association of the human papillomavirus type 11 E1()E4 protein with cornified cell envelopes derived from infected genital epithelium. Virology 277:262-269. - PubMed
-
- Chan, T. A., H. Hermeking, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1999. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature 401:616-620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

