Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts
- PMID: 15767406
- PMCID: PMC1061583
- DOI: 10.1128/JVI.79.7.4043-4054.2005
Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts
Abstract
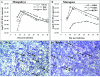
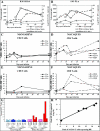
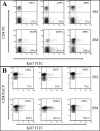
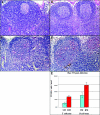
To understand how natural sooty mangabey hosts avoid AIDS despite high levels of simian immunodeficiency virus (SIV) SIVsm replication, we inoculated mangabeys and nonnatural rhesus macaque hosts with an identical inoculum of uncloned SIVsm. The unpassaged virus established infection with high-level viral replication in both macaques and mangabeys. A species-specific, divergent immune response to SIV was evident from the first days of infection and maintained in the chronic phase, with macaques showing immediate and persistent T-cell proliferation, whereas mangabeys displayed little T-cell proliferation, suggesting subdued cellular immune responses to SIV. Importantly, only macaques developed (CD4+)-T-cell depletion and AIDS, thus indicating that in mangabeys limited immune activation is a key mechanism to avoid immunodeficiency despite high levels of SIVsm replication. These studies demonstrate that it is the host response to infection, rather than properties inherent to the virus itself, that causes immunodeficiency in SIV-infected nonhuman primates.
Figures



References
-
- Carlson, J. R., T. P. McGraw, E. Keddie, J. L. Yee, A. Rosenthal, A. J. Langlois, R. Dickover, R. Donovan, P. A. Luciw, and M. B. Jennings. 1990. Vaccine protection of rhesus macaques against simian immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 6:1239-1246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

