Cooperative effect of gag proteins p12 and capsid during early events of murine leukemia virus replication
- PMID: 15767417
- PMCID: PMC1061564
- DOI: 10.1128/JVI.79.7.4159-4169.2005
Cooperative effect of gag proteins p12 and capsid during early events of murine leukemia virus replication
Abstract
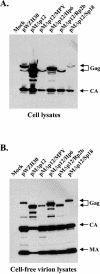
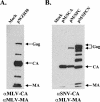
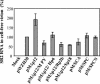
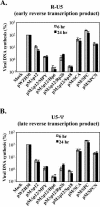
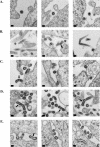
The Gag polyprotein of murine leukemia virus (MLV) is processed into matrix (MA), p12, capsid (CA), and nucleocapsid (NC) proteins. p12 affects early events of virus replication and contains a PPPY motif important for virus release. To probe the functions of p12 in the early steps of MLV replication, we tested whether p12 can be replaced by spleen necrosis virus (SNV) p18, human immunodeficiency virus type 1 p6, or Rous sarcoma virus p2b. Analyses revealed that all chimeras generated virions at levels similar to that of MLV gag-pol; however, none of them could support MLV vector replication, and all of them exhibited severely reduced DNA synthesis upon virus infection. Because a previously reported SNV gag-MLV pol chimera, but not the MLV hybrid with SNV p18, can support replication of an MLV vector, we hypothesized that other Gag proteins act cooperatively with p12 during the early phase of virus replication. To test this hypothesis, we generated three more MLV-based chimeras containing SNV CA, p18-CA, or p18-CA-NC. We found that the MLV chimera containing SNV p18-CA or p18-CA-NC could support MLV vector replication, but the chimera containing SNV CA could not. Furthermore, viruses derived from the MLV chimera with SNV CA could synthesize viral DNA upon infection but were blocked at a post-reverse-transcription step and generated very little two long terminal repeat circle DNA, thereby producing a phenotype similar to that of the provirus formation-defective p12 mutants. Taken together, our data indicate that when p12/p18 or CA was from different viruses, despite abundant virus production and proper Gag processing, the resulting viruses were not infectious. However, when p12/p18 and CA were from the same virus, even though they were from SNV and not MLV, the resulting viruses were infectious. Therefore, these results suggest a cooperative effect of p12 and CA during the early events of MLV replication.
Figures


Similar articles
-
The nucleocapsid domain is responsible for the ability of spleen necrosis virus (SNV) Gag polyprotein to package both SNV and murine leukemia virus RNA.J Virol. 1999 Nov;73(11):9170-7. doi: 10.1128/JVI.73.11.9170-9177.1999. J Virol. 1999. PMID: 10516024 Free PMC article.
-
Mutant murine leukemia virus Gag proteins lacking proline at the N-terminus of the capsid domain block infectivity in virions containing wild-type Gag.Virology. 2006 Apr 10;347(2):364-71. doi: 10.1016/j.virol.2005.12.012. Epub 2006 Jan 19. Virology. 2006. PMID: 16427108
-
Capsid is an important determinant for functional complementation of murine leukemia virus and spleen necrosis virus Gag proteins.Virology. 2007 Apr 10;360(2):388-97. doi: 10.1016/j.virol.2006.10.038. Epub 2006 Dec 6. Virology. 2007. PMID: 17156810 Free PMC article.
-
The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance.Int J Biochem Cell Biol. 2004 Sep;36(9):1668-86. doi: 10.1016/j.biocel.2004.02.024. Int J Biochem Cell Biol. 2004. PMID: 15183337 Review.
-
The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance.Curr HIV Res. 2004 Jan;2(1):79-92. doi: 10.2174/1570162043485022. Curr HIV Res. 2004. PMID: 15053342 Review.
Cited by
-
Interaction of moloney murine leukemia virus capsid with Ubc9 and PIASy mediates SUMO-1 addition required early in infection.J Virol. 2006 Jan;80(1):342-52. doi: 10.1128/JVI.80.1.342-352.2006. J Virol. 2006. PMID: 16352559 Free PMC article.
-
Phosphorylation Requirement of Murine Leukemia Virus p12.J Virol. 2016 Nov 28;90(24):11208-11219. doi: 10.1128/JVI.01178-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27707931 Free PMC article.
-
The p12 domain is unstructured in a murine leukemia virus p12-CA(N) Gag construct.PLoS One. 2008 Apr 2;3(4):e1902. doi: 10.1371/journal.pone.0001902. PLoS One. 2008. PMID: 18382677 Free PMC article.
-
The gammaretroviral p12 protein has multiple domains that function during the early stages of replication.Retrovirology. 2012 Oct 4;9:83. doi: 10.1186/1742-4690-9-83. Retrovirology. 2012. PMID: 23035841 Free PMC article.
-
ESCRT requirements for EIAV budding.Retrovirology. 2013 Oct 9;10:104. doi: 10.1186/1742-4690-10-104. Retrovirology. 2013. PMID: 24107264 Free PMC article.
References
-
- Alin, K., and S. P. Goff. 1996. Amino acid substitutions in the CA protein of Moloney murine leukemia virus that block early events in infection. Virology 222:339-351. - PubMed
-
- Bowerman, B., P. O. Brown, J. M. Bishop, and H. E. Varmus. 1989. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 3:469-478. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

