Human papillomavirus type 18 E6 protein binds the cellular PDZ protein TIP-2/GIPC, which is involved in transforming growth factor beta signaling and triggers its degradation by the proteasome
- PMID: 15767424
- PMCID: PMC1061528
- DOI: 10.1128/JVI.79.7.4229-4237.2005
Human papillomavirus type 18 E6 protein binds the cellular PDZ protein TIP-2/GIPC, which is involved in transforming growth factor beta signaling and triggers its degradation by the proteasome
Abstract
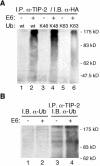
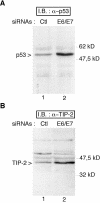
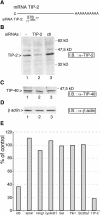
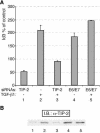
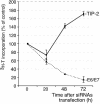
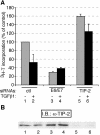
Several viral proteins expressed by DNA or RNA transforming viruses have the particular property of binding via their C-terminal end to various cellular proteins with PDZ domains. This study is focused on the PDZ protein TIP-2/GIPC, which was originally identified in two-hybrid screens performed with two different baits: the human T-cell leukemia virus type 1 Tax oncoprotein and the regulator of G signaling RGS-GAIP. Further studies have shown that TIP-2/GIPC is also able to associate with the cytoplasmic domains of various transmembrane proteins. In this report we show that TIP-2/GIPC interacts with the E6 protein of human papillomavirus type 18 (HPV-18). This event triggers polyubiquitination and proteasome-mediated degradation of the cellular protein. In agreement with this observation, silencing of E6 by RNA interference in HeLa cells causes an increase in the intracellular TIP-2/GIPC level. This PDZ protein has been previously found to be involved in transforming growth factor beta (TGF-beta) signaling by favoring expression of the TGF-beta type III receptor at the cell membrane. In line with this activity of TIP-2/GIPC, we observed that depletion of this protein in HeLa cells hampers induction of the Id3 gene by TGF-beta treatment and also diminishes the antiproliferative effect of this cytokine. Conversely, silencing of E6 increases the expression of Id3 and blocks proliferation of HeLa cells. These results support the notion that HPV-18 E6 renders cells less sensitive to the cytostatic effect of TGF-beta by lowering the intracellular amount of TIP-2/GIPC.
Figures

References
-
- Awan, A., M. R. Lucic, D. M. Shaw, F. Sheppard, C. Westwater, S. A. Lyons, and P. L. Stern. 2002. 5T4 interacts with TIP-2/GIPC, a PDZ protein, with implications for metastasis. Biochem. Biophys. Res. Commun. 290:1030-1036. - PubMed
-
- Blobe, G. C., X. Liu, S. J. Fang, T. How, and H. F. Lodish. 2001. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 276:39608-39617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

