Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport
- PMID: 15767438
- PMCID: PMC1061540
- DOI: 10.1128/JVI.79.7.4382-4395.2005
Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport
Abstract
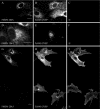
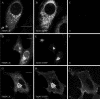
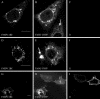
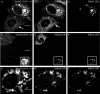
Infection of cells by picornaviruses leads to the generation of intracellular membrane vesicles. The expression of poliovirus (PV) 3A protein causes swelling of the endoplasmic reticulum (ER) and inhibition of protein trafficking between the ER and the Golgi apparatus. Here, we report that the nonstructural proteins of a second picornavirus, foot-and-mouth disease virus (FMDV), also perturb the secretory pathway. FMDV proteins 3A, 2B, 2C, and 2BC expressed alone in cells were recovered from crude membrane fractions, indicating membrane association. Immunofluorescence microscopy showed that 3A was located in a reticular structure and 2B was located in the ER, while 2C was located in both the ER and the bright punctate structures within the Golgi apparatus. 2BC gave punctate cytoplasmic staining and also caused accumulation of ER proteins in large vesicular structures located around the nuclei. The effect of the FMDV proteins on the trafficking of the vesicular stomatitis virus glycoprotein (G protein) from the ER to the cell surface was determined. Unlike its PV counterpart, the 3A protein of FMDV did not prevent trafficking of the G protein to the cell surface. Instead, surface expression of the G protein was blocked by 2BC, with retention of the G protein in a modified ER compartment staining for 2BC. The results suggest that the nonstructural proteins of different picornaviruses may vary in their ability to perturb the secretory pathway. Since FMDV 2BC can block the delivery of proteins to the cell surface, it may, as shown for PV 3A, play a role in immune evasion and contribute to the persistent infections observed in ruminants.
Figures

Similar articles
-
Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C.J Virol. 2007 Feb;81(3):1129-39. doi: 10.1128/JVI.00393-06. Epub 2006 Nov 22. J Virol. 2007. PMID: 17121791 Free PMC article.
-
Differential distribution of non-structural proteins of foot-and-mouth disease virus in BHK-21 cells.Virology. 2006 Jun 5;349(2):409-21. doi: 10.1016/j.virol.2006.02.042. Epub 2006 Apr 19. Virology. 2006. PMID: 16624365
-
Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus.Eur J Cell Biol. 1990 Dec;53(2):185-96. Eur J Cell Biol. 1990. PMID: 1964413
-
Maintenance of Golgi apparatus structure in the face of continuous protein recycling to the endoplasmic reticulum: making ends meet.Int Rev Cytol. 2005;244:69-94. doi: 10.1016/S0074-7696(05)44002-4. Int Rev Cytol. 2005. PMID: 16157178 Review.
-
Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen.FEMS Immunol Med Microbiol. 2008 Jun;53(1):8-17. doi: 10.1111/j.1574-695X.2008.00409.x. Epub 2008 Apr 8. FEMS Immunol Med Microbiol. 2008. PMID: 18400012 Review.
Cited by
-
Foot-and-mouth disease virus 3C protease induces fragmentation of the Golgi compartment and blocks intra-Golgi transport.J Virol. 2013 Nov;87(21):11721-9. doi: 10.1128/JVI.01355-13. Epub 2013 Aug 28. J Virol. 2013. PMID: 23986596 Free PMC article.
-
Molecular Characterization of the Viroporin Function of Foot-and-Mouth Disease Virus Nonstructural Protein 2B.J Virol. 2018 Nov 12;92(23):e01360-18. doi: 10.1128/JVI.01360-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232178 Free PMC article.
-
Viral infection: Moving through complex and dynamic cell-membrane structures.Commun Integr Biol. 2011 Jul;4(4):398-408. doi: 10.4161/cib.16716. Epub 2011 Jul 1. Commun Integr Biol. 2011. PMID: 21966556 Free PMC article.
-
Biological function of Foot-and-mouth disease virus non-structural proteins and non-coding elements.Virol J. 2016 Jun 22;13:107. doi: 10.1186/s12985-016-0561-z. Virol J. 2016. PMID: 27334704 Free PMC article. Review.
-
Foot-and-mouth disease virus induces autophagosomes during cell entry via a class III phosphatidylinositol 3-kinase-independent pathway.J Virol. 2012 Dec;86(23):12940-53. doi: 10.1128/JVI.00846-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993157 Free PMC article.
References
-
- Aldabe, R., A. Barco, and L. Carrasco. 1996. Membrane permeabilization by poliovirus proteins 2B and 2BC. J. Biol Chem. 271:23134-23137. - PubMed
-
- Alexandersen, S., Z. Zhang, and A. I. Donaldson. 2002. Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. Microbes Infect. 4:1099-1110. - PubMed
-
- Barlowe, C., L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M. F. Rexach, M. Ravazzola, M. Amherdt, and R. Schekman. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77:895-907. - PubMed
-
- Bergmann, J. E. 1989. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol. 32:85-110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

