The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus
- PMID: 15767446
- PMCID: PMC1061561
- DOI: 10.1128/JVI.79.7.4460-4469.2005
The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus
Abstract
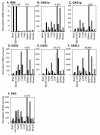
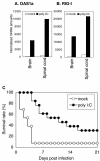
Poliovirus selectively replicates in neurons in the spinal cord and brainstem, although poliovirus receptor (PVR) expression is observed in both the target and nontarget tissues in humans and transgenic mice expressing human PVR (PVR-transgenic mice). We assessed the role of alpha/beta interferon (IFN) in determining tissue tropism by comparing the pathogenesis of the virulent Mahoney strain in PVR-transgenic mice and PVR-transgenic mice deficient in the alpha/beta IFN receptor gene (PVR-transgenic/Ifnar knockout mice). PVR-transgenic/Ifnar knockout mice showed increased susceptibility to poliovirus. After intravenous inoculation, severe lesions positive for the poliovirus antigen were detected in the liver, spleen, and pancreas in addition to the central nervous system. These results suggest that the alpha/beta IFN system plays an important role in determining tissue tropism by protecting nontarget tissues that are potentially susceptible to infection. We subsequently examined the expression of IFN and IFN-stimulated genes (ISGs) in the PVR-transgenic mice. In the nontarget tissues, ISGs were expressed even in the noninfected state, and the expression level increased soon after poliovirus infection. On the contrary, in the target tissues, ISG expression was low in the noninfected state and sufficient response after poliovirus infection was not observed. The results suggest that the unequal IFN response is one of the important determinants for the differential susceptibility of tissues to poliovirus. We consider that poliovirus replication was observed in the nontarget tissues of PVR-transgenic/Ifnar knockout mice because the IFN response was null in all tissues.
Figures


References
-
- Asada-Kubota, M., T. Ueda, M. Shimada, K. Takeda, and T. Sokawa. 1995. Distribution of immunoreactive 2′, 5′-oligoadenylate synthetase in mouse digestive tract. J. Interferon Cytokine Res. 15:863-867. - PubMed
-
- Bodian, D. 1949. Histopathologic basis of clinical findings in poliomyelitis. Am. J. Med. 6:563-578. - PubMed
-
- Bodian, D. 1954. Viremia in experimental poliomyelitis. I. General aspects of infection after intravascular inoculation with strains of high and of low invasiveness. Am. J. Hyg. 60:339-357. - PubMed
-
- Bodian, D. 1955. Emerging concept of poliomyelitis infection. Science 12:105-108. - PubMed
-
- Bodian, D. 1956. Poliovirus in chimpanzee tissues after virus feeding. Am. J. Hyg. 64:181-197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

