Mechanism of the antiviral action of 1-beta-D-arabinofuranosylcytosine on Borna disease virus
- PMID: 15767452
- PMCID: PMC1061581
- DOI: 10.1128/JVI.79.7.4514-4518.2005
Mechanism of the antiviral action of 1-beta-D-arabinofuranosylcytosine on Borna disease virus
Abstract
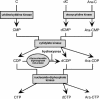
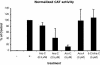
Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus that causes neurological diseases in a variety of warm-blooded animal species. Recently, we showed that the nucleoside analog 1-beta-D-arabinofuranosylcytosine (Ara-C) was a potent inhibitor of BDV. This finding was surprising for an RNA virus, since Ara-C is a DNA polymerase inhibitor. Thus, we sought to better define the mechanism of action of Ara-C on BDV. Here, we show that (i) this effect is specific for an arabinoside ring carrying a cytosine base, (ii) it requires phosphorylation of the nucleotide, and (iii) it can be reversed by an excess of cytidine. Using the recently described minigenome assay for BDV, we provide evidence suggesting that Ara-C may act as a competitive inhibitor of the BDV replication complex.
Figures

Similar articles
-
1-beta-D-arabinofuranosylcytosine inhibits borna disease virus replication and spread.J Virol. 2002 Jun;76(12):6268-76. doi: 10.1128/jvi.76.12.6268-6286.2002. J Virol. 2002. PMID: 12021360 Free PMC article.
-
2'-fluoro-2'-deoxycytidine inhibits Borna disease virus replication and spread.Antimicrob Agents Chemother. 2004 Apr;48(4):1422-5. doi: 10.1128/AAC.48.4.1422-1425.2004. Antimicrob Agents Chemother. 2004. PMID: 15047559 Free PMC article.
-
MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells.J Virol. 2001 May;75(10):4871-7. doi: 10.1128/JVI.75.10.4871-4877.2001. J Virol. 2001. PMID: 11312358 Free PMC article.
-
Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus.Virus Res. 2005 Aug;111(2):148-60. doi: 10.1016/j.virusres.2005.04.006. Virus Res. 2005. PMID: 15992626 Review.
-
Borna disease: current knowledge and virus detection in France.Vet Res. 2002 Mar-Apr;33(2):127-38. doi: 10.1051/vetres:2002002. Vet Res. 2002. PMID: 11944803 Review.
Cited by
-
Borna disease virus phosphoprotein modulates epigenetic signaling in neurons to control viral replication.J Virol. 2015 Jun;89(11):5996-6008. doi: 10.1128/JVI.00454-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810554 Free PMC article.
References
-
- Bhalla, K., P. Swerdlow, and S. Grant. 1991. Effects of thymidine and hydroxyurea on the metabolism and cytotoxicity of 1-β-d arabinofuranosylcytosine in highly resistant human leukemia cells. Blood 78:2937-2944. - PubMed
-
- Bode, L., D. E. Dietrich, R. Stoyloff, H. M. Emrich, and H. Ludwig. 1997. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet 349:178-179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

