Lack of heme synthesis in a free-living eukaryote
- PMID: 15767563
- PMCID: PMC555530
- DOI: 10.1073/pnas.0500877102
Lack of heme synthesis in a free-living eukaryote
Abstract
In most free-living eukaryotes studied thus far, heme is synthesized from a series of intermediates through a well defined evolutionarily conserved pathway. We found that free-living worms, including the model genetic organism Caenorhabditis elegans, and parasitic helminths are unable to synthesize heme de novo, even though these animals contain hemoproteins that function in key biological processes. Radioisotope, fluorescence labeling, and heme analog studies suggest that C. elegans acquires heme from exogenous sources. Iron-deprived worms were unable to grow in the presence of adequate heme unless rescued by increasing heme levels in the growth medium. These data indicate that although worms use dietary heme for incorporation into hemoproteins, ingested heme is also used as an iron source when iron is limiting. Our results provide a biochemical basis for the dependence of worm growth and development on heme, and they suggest that pharmacologic targeting of heme transport pathways in worms could be an important control measure for helminthic infections.
Figures

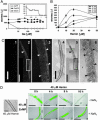
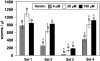
Similar articles
-
Lessons from bloodless worms: heme homeostasis in C. elegans.Biometals. 2015 Jun;28(3):481-9. doi: 10.1007/s10534-015-9841-0. Epub 2015 Feb 28. Biometals. 2015. PMID: 25724951 Free PMC article.
-
Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins.Nature. 2008 Jun 19;453(7198):1127-31. doi: 10.1038/nature06934. Epub 2008 Apr 16. Nature. 2008. PMID: 18418376 Free PMC article.
-
Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2.J Biol Chem. 2012 Mar 16;287(12):9601-12. doi: 10.1074/jbc.M111.307694. Epub 2012 Feb 2. J Biol Chem. 2012. PMID: 22303006 Free PMC article.
-
Notes from the Underground: Heme Homeostasis in C. elegans.Biomolecules. 2023 Jul 19;13(7):1149. doi: 10.3390/biom13071149. Biomolecules. 2023. PMID: 37509184 Free PMC article. Review.
-
Metabolic engineering of yeast to efficiently synthesize heme and hemoproteins: recent advance and prospects.FEMS Yeast Res. 2025 Jan 30;25:foaf019. doi: 10.1093/femsyr/foaf019. FEMS Yeast Res. 2025. PMID: 40228812 Free PMC article. Review.
Cited by
-
Regulation of intracellular heme trafficking revealed by subcellular reporters.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):E5144-52. doi: 10.1073/pnas.1609865113. Epub 2016 Aug 15. Proc Natl Acad Sci U S A. 2016. PMID: 27528661 Free PMC article.
-
Aerobic kinetoplastid flagellate Phytomonas does not require heme for viability.Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3808-13. doi: 10.1073/pnas.1201089109. Epub 2012 Feb 21. Proc Natl Acad Sci U S A. 2012. PMID: 22355128 Free PMC article.
-
Heme pathway evolution in kinetoplastid protists.BMC Evol Biol. 2016 May 18;16(1):109. doi: 10.1186/s12862-016-0664-6. BMC Evol Biol. 2016. PMID: 27193376 Free PMC article.
-
A Transparent Window into Biology: A Primer on Caenorhabditis elegans.Genetics. 2015 Jun;200(2):387-407. doi: 10.1534/genetics.115.176099. Genetics. 2015. PMID: 26088431 Free PMC article.
-
Wide diversity in structure and expression profiles among members of the Caenorhabditis elegans globin protein family.BMC Genomics. 2007 Oct 4;8:356. doi: 10.1186/1471-2164-8-356. BMC Genomics. 2007. PMID: 17916248 Free PMC article.
References
-
- Colley, D. G., LoVerde, P. T. & Savioli, L. (2001) Science 293, 1437–1438. - PubMed
-
- Chitwood, D. J. (2003) Pest Manage. Sci. 59, 748–753. - PubMed
-
- Sangster, N. C. & Gill, J. (1999) Parasitol. Today 15, 141–146. - PubMed
-
- Ismail, M., Botros, S., Metwally, A., William, S., Farghally, A., Tao, L. F., Day, T. A. & Bennett, J. L. (1999) Am. J. Trop. Med. Hyg. 60, 932–935. - PubMed
-
- Halton, D. W. (1997) Int. J. Parasitol. 27, 693–704. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

